SAN DIEGO—In a session titled, In Vivo Administration of MiR-146a Protects C57BL/6 Mice from Pristane-Induced Pulmonary Hemorrhage via Suppressing Type 1 Interferon Response, Nan Shen, MD, professor of medicine and director, Shanghai Institute of Rheumatology, Renji Hospital, Jiao Tong University School of Medicine, Shanghai, China, presented the Rheumatology Research Foundation Edmond L. Dubois, MD, Memorial Lectureship at the 2013 ACR/ARHP Annual Meeting. (Editor’s note: This session was recorded and is available via ACR SessionSelect at www.rheumatology.org.)
Along with reviewing previous studies that have shown the crucial role of microRNAs (miRNAs) for human lupus, and in particular the role of a novel functional variant in the microRNA-146a gene, Dr. Shen presented findings from a current study that explored a therapeutic potential of miRNA-146a in a murine model of lupus.
“Growing evidence shows deregulation and dysfunction of miRNAs in human lupus and lupus-like mice model, and that miRNAs are associated with prognosis and progression of several human diseases and may serve as future targets for gene therapy,” Dr. Shen said. “Indeed, the clear importance of miRNA to disease expression is striking in both man and mouse, and several clinical trials are now underway using [a] miRNA inhibition approach for treating several common diseases.”
MicroRNAs—Some Background
“MicroRNAs represent a large class of small non-coding RNAs that ubiquitously exist in a broad range of species from worms to mammals, which function as an important class of molecular mediators functioning in post-transcriptional regulation, [and] play a crucial role in diverse biological processes, including regulation of immune response and involvement in autoimmunity,” said Dr. Shen.
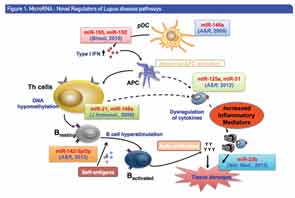
Figure 1 illustrates miRNAs as novel regulators of the disease pathways of lupus. In his talk, Dr. Shen provided data from previous studies he and his colleagues have done to dissect the molecular mechanisms underlining the genetic and epigenetic control of lupus development. In these studies, they were able to show that overexpression of miRNA-146a in the peripheral blood mononucleated cells (PBMCs) from patients with systemic lupus erythematosus (SLE) suppressed activation of the type 1 interferon (IFN) pathway.1,2,3 This finding suggested to Dr. Shen and his colleagues that manipulation of miRNA-146a levels may be a useful therapeutic intervention in SLE, and prompted the current study to investigate the therapeutic potential of miRNA-146a for lupus intervention by an induced murine model of SLE.
Study: Therapeutic Potential of miR-146a in Induced Murine Model of Lupus
In their current study, Shen and colleagues used pristane-induced lupus, a well-established SLE murine model featuring abnormal activation of IFN pathway and an unusually high incidence of pulmonary capillaritis. Mice were intravenously injected with MiRNA-146a or phosphate-buffered saline (PBS) via lateral tail veins. Real-time polymerase chain reaction (PCR) or gene expression profile analysis was used to evaluate the peripheral blood drawn 3, 7 and 14 days after pristane injection. Mice were categorized into three groups for comparison: those receiving three consecutive injections of PBS (control group; n=9), those receiving miRNA-146a three days before a single intraperitoneal injection of pristane (prevention group; n=19), and those receiving miRNA-146a seven days after a single intraperitoneal injection of pristane (treatment group; n=10). The development of pulmonary hemorrhage was confirmed two weeks after the pristane injection using a hematoxylin and eosin (HE) stain.
The study found a dramatic increase in the expression of peripheral blood miRNA-146a in both the prevention and treatment groups in mice injected with miRNA-146a compared with the mice in the control group, and HE staining showed that the administration of miRNA-146a rendered mice resistant to pristane-induced hemorrhagic pulmonary capillaritis. The prevalence of pulmonary hemorrhage was reduced to 25% in mice in the treatment group, and completely blocked in the prevention group. In the mice in the control group, 56% injected with pristane developed complete pulmonary hemorrhage. In addition, the study found that mice with pulmonary hemorrhage had significantly lower miRNA-146a compared with mice that did not develop hemorrhage (P<0.01), and that miRNA-146a injection substantially suppressed the IFN response and reduced production of multiple proinflammatory cytokines and chemokines.
“Our study provides evidence that miRNA-146a plays a suppressive role in the pristane-induced pulmonary hemorrhage in B6 mice, and highlights a potential pathogenic role of IFN-I pathway activation in the development of pulmonary capillaritis in pristane-induced murine lupus,” said Dr. Shen. “These findings suggest that SLE patients with pulmonary hemorrhage may benefit from therapeutic intervention to induce miRNA-146a expression.”
Overall, Dr. Shen said he is hoping that the results of this study and previous investigation into the role of miRNAs will demonstrate the impressive progress made using miRNA therapeutics in mouse studies. “At an early stage, miRNA-based therapeutics hold enormous promise for reversing inflammatory consequences of disease,” he said, adding that “approaches to enhance or interrupt miRNAs function may have therapeutic utility, as well as revealing molecular and cellular mechanisms responsible for lupus onset and progression.”
Mary Beth Nierengarten is a freelance medical journalist based in St. Paul, Minn.
References
- Shen N, Liang D, Tang Y, et al. MicroRNAs—novel regulators of systemic lupus erythematosus pathogenesis. Nat Rev Rheumatol. 2012;8(12):701–709.
- Tang Y, Luo X, Cui H, et al. MicroRNA-146A contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum. 2009;60(4):1065–1075.
- Luo XB, Yang W, Ye D-Q, et al. A functional variant in MicroRNA-146a promoter modulates its expression and confers disease risk for systemic lupus erythematosus. PLoS Genet. 2011;7(6):e1002128. Epub 2011 Jun 30.