In his new role as the 86th president of the ACR, Douglas White, MD, PhD, is excited about the opportunities to serve and energize the organization and members in the coming year.


Leslie Mertz, PhD |
In his new role as the 86th president of the ACR, Douglas White, MD, PhD, is excited about the opportunities to serve and energize the organization and members in the coming year.

Richard L. Allman, MD, MS, FACP, FACR |
The patient, a 76-year-old woman, had very active polyarticular rheumatoid arthritis (RA), despite triple therapy with conventional synthetic disease-modifying anti-rheumatic drugs (DMARDs), low-dose corticosteroids and occasional intra-articular injections—the latter providing only transient symptomatic relief. She had elevated inflammatory markers and a 28-joint Disease Activity Score (DAS-28) score of 7.4. Because of the severity of her…

Catherine Kolonko |
Early in 2022, a few months into the invasion of Ukraine by Russian troops, Paula Rackoff, MD, a rheumatologist and clinical associate professor at NYU Grossman School of Medicine, New York, felt an urgency to head to the region to assist the many refugees fleeing for the border with Poland. Dr. Rackoff canceled a planned…
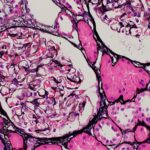
Matthew J. Mandell, DO, Yishui Chen, MD, Prerna Rastogi, MD, PhD, & Rebecca Tuetken, MD, PhD |
Syphilis, an ancient disease caused by the spirochete Treponema pallidum, has been historically referred to as the great mimicker given its heterogenous presentation. Both systemic lupus erythematosus (SLE) and syphilis can have multi-systemic involvement. Both parvovirus B19 and syphilis have been reported to cause histologic features similar to those seen in lupus nephritis. We present…

Despite an expanding armamentarium of disease-modifying treatments for rheumatoid arthritis (RA), some patients with RA remain symptomatic.1 Current treatment guidelines from both the ACR and the European Alliance of Associations for Rheumatology (EULAR) recommend treat-to-target strategies to achieve remission or low disease activity, and patients want to feel better.2,3 So how can we best help…

Rheumatologists who are outstanding clinicians, provide consistently exceptional care to patients and serve as role models for colleagues and trainees are in the spotlight in our Lessons from a Master Clinician series. Here, we offer insights from clinicians who have achieved a level of distinction in the field of rheumatology. Eric L. Matteson, MD, MPH,…
Rochelle Castillo, MD, MS, Andro Licaros, MD, & Jemima Albayda, MD |
In medicine, as in advertising, pictures can be worth a thousand words. From arthritis to vasculitis, imaging studies have been variably employed to aid in the diagnosis, treatment, risk stratification and prognostication of patients with rheumatic and musculoskeletal disorders. The same holds true with the idiopathic inflammatory myopathies (IIM), in which the clinical utility is…

Charles Radis, DO |
On a highway traversed by cement trucks and Beetle-Bug auto-rickshaws we travel north from Bangalore, India, for a house call. It is 2007, and the city leaves us grudgingly. Between fields of loose chocolate soil and sprigs of beans poking skyward, the skeletons of homes and businesses rise; armies of workers lay brick from wooden…

“Fifty percent of kids with rheumatic disease are taken care of by adult providers,” says Jay J. Mehta, MD, MS, attending physician and fellowship program director, Department of Rheumatology, Children’s Hospital of Philadelphia, and a co-author of the ACR’s recent pediatric workforce shortage study.1,2 “But adult rheumatologists may not have specific training in the rheumatic…

Oshrat E. Tayer-Shifman, MD; Kimberley Yuen, BSc, MD; Zahi Touma, MD, PhD, FACP, FACR; William Daniel Soulsby, MD; Aleksandra Kostic, BSE; Valia Leifer, MA; & Elena Losina, PhD, MSC |
MoCA as a Screening Test in SLE Assessing the utility of the Montreal Cognitive Assessment (MoCA) By Oshrat E. Tayer-Shifman, MD, Kimberley Yuen, BSc, MD, & Zahi Touma, MD, PhD, FACP, FACR Why was this study done? Cognitive impairment is a common manifestation of systemic lupus erythematosus (SLE), with a prevalence of 40% based on…