 Inebilizumab reduces the risk of immunoglobulin G4-related disease (IgG4-RD) flares and increases the likelihood of flare-free, complete remission at one year, a recent study shows.1 The randomized, doubleblind, placebo-controlled MITIGATE trial showed that inebilizumab reduced the risk of IgG4-RD symptoms by 87%, compared with placebo.
Inebilizumab reduces the risk of immunoglobulin G4-related disease (IgG4-RD) flares and increases the likelihood of flare-free, complete remission at one year, a recent study shows.1 The randomized, doubleblind, placebo-controlled MITIGATE trial showed that inebilizumab reduced the risk of IgG4-RD symptoms by 87%, compared with placebo.
About IgG4-RD
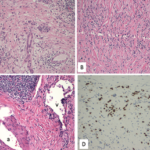
IgG4-RD is chronic, rare condition that has, so far, been estimated to affect fewer than 200,000 people in the U.S. The systemic, autoimmune inflammatory disease leads to lesions rich in CD19+ B cells. These cells are thought to directly drive inflammation, and scarring and thickening of tissue directly through cytokines, proteins that affect the immune system. The cells may also indirectly activate pathogenic T cells.
Patients with IgG4-RD suffer from the build-up of immune cells, which produce the IgG4 antibody in certain organs. Commonly affected organs include the pancreas, bile ducts, salivary glands, abdominal tissue, eyes and lungs. Symptoms vary, depending on the affected organ. For example, the disease commonly affects the pancreas, causing pancreatitis and diabetes. When the disease affects eye tissues, patients may suffer double vision. Often multiple organs are affected. Many patients undergo a long diagnostic journey and are initially misdiagnosed, some even undergoing unnecessary surgery.
Currently, there is no approved drug for IgG4-RD. The usual, standard treatment for IgG4-RD is prednisone, which reduces disease flares but is problematic.
“Prednisone is toxic for patients, who tend to be middle aged or elderly at the time they’re diagnosed. This is compounded by the fact that IgG4-RD patients often have pancreatic problems—either endocrine or exocrine insufficiency or both—because of the tendency of the disease to target the pancreas,” says the study’s first author, John H. Stone, MD, MPH, a professor of medicine at Harvard Medical School, Boston, and a rheumatologist in the Division of Rheumatology, Allergy, and Immunology at Massachusetts General Hospital (MGH), Boston. “That standard of care [may] be replaced by a medication that will allow patients to be on essentially no steroids after a very short period of time.”
Inebilizumab, manufactured by Amgen, the study’s sponsor, depletes CD19-expressing B cells. The biologic is a lab-created protein designed to mimic a naturally occurring antibody to target the CD-19 antigen.
The Study
The phase 3 MITIGATE trial examined the efficacy and safety of inebilizumab in reducing flare risk for adults with IgG4-RD. The researchers studied 135 participants who were randomized one to one to receive 300 mg of intravenous inebilizumab or placebo on days 1 and 15, and at week 26 after premedication. The participants were followed for a 52-week randomized control period.