About 30% of patients with psoriasis have psoriatic arthritis (PsA), a complex, multi-faceted, chronic, inflammatory musculoskeletal and skin disease for which the treatment has changed considerably over the past few years.1 Biosimilars and other new drugs have become a therapeutic turning point for many patients suffering from rheumatic illnesses, including PsA. The treatment of PsA includes a variety of options, some of which are off-label therapies.
In 2019, the ACR and the National Psoriasis Foundation (NPF) published their first joint guideline for treating psoriatic arthritis. Review it at https://www.rheumatology.org/Practice-Quality/Clinical-Support/Clinical-Practice-Guidelines/Psoriatic-Arthritis.2 The ACR/NPF guideline defined active PsA based on activity in any features (i.e., actively inflamed joints, dactylitis, enthesitis, axial disease, active skin and/or nail involvement, and/or extra-articular manifestations, such as uveitis or inflammatory bowel disease), based on whether the manifestations are clinically significant and attributable to psoriatic arthritis. Although the impact of PsA on quality of life is comparable to that of rheumatoid arthritis (RA), significantly fewer resources exist for patients living with PsA than for patients with RA.3 The disparities in health resources for PsA include:
- Limited research;
- Misunderstood symptoms or inadequate treatment;
- Delayed diagnoses;
- Lack of understanding of how the disease can impact quality of life; and
- Conflicting information that impacts the management of the disease.
Disease Management Decision
The ACR/NPF guideline was created to assist healthcare providers and their patients in making challenging disease management decisions and select optimal therapy. Although the number of new therapies available for the treatment of PsA has increased, limited comparative efficacy and effectiveness evidence exists to inform treatment decisions.
The treat-to-target approach started as a strategy for managing heart disease and diabetes and is now the most widely accepted treatment approach for RA and other inflammatory diseases.4 However, treat to target is not the standard of care in PsA; it is a conditional recommendation, based on low- to very-low-quality evidence, in the ACR/NPF guideline.
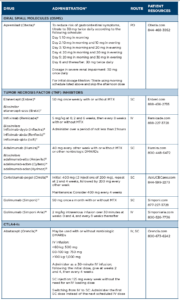
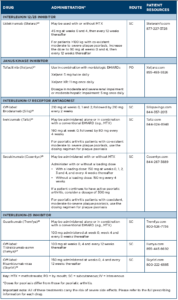
Treatments for PsA include traditional or conventional disease-modifying anti-rheumatic drugs (DMARDs); biologic therapies, such as tumor necrosis factor (TNF) inhibitors, interleukin (IL) 17 receptor antagonists, and IL-12 and IL-23 inhibitors; and targeted new oral agents, including a phosphodiesterase-4 inhibitor (PDE4) and a Janus kinase (JAK) inhibitor. Additional therapies approved for psoriasis but not yet approved for PsA include an IL-17 receptor antagonist (brodalumab) and two IL-23 inhibitors (tildrakizumab and risankizumab). (Note: For detailed info for each drug, see Table 1 and Table 2 below.)
In addition to pharmacologic recommendations, the ACR/NPF guideline conditionally recommends the following non-pharmacologic therapies: low-impact exercise (e.g., tai chi, yoga, swimming), physical therapy, occupational therapy, weight loss in patients who are overweight or obese, massage and acupuncture. Smoking cessation was strongly recommended because it is associated with cardiovascular disease and worse treatment outcomes.
Summary by Drug Class
Drug Class: PDE4 inhibitor
Drug: apremilast (Otezla)5
Adverse reactions: The most common adverse reactions (≥5%) are diarrhea, nausea and headache.
Drug Class: TNF inhibitors6-18
Drugs: etanercept (Enbrel), infliximab (Remicade), adalimumab (Humira), certolizumab pegol (Cimzia), golimumab injection for subcutaneous use (Simponi), golimumab injection for intravenous use (Simponi Aria)
Biosimilars: etanercept-szzs (Erelzi), infliximab-dyyb (Inflectra), infliximababda (Renflexis), infliximab-qbtx (Ixifi), adalimumab-atto (Amjevita), adalimumab-adbm (Cyltezo), adalimumab-adaz (Hyrimoz)
Boxed Warning: Serious Infections & Malignancies
- TNF inhibitors have an increased risk of serious infections leading to hospitalization or death, including tuberculosis (TB), bacterial sepsis, invasive fungal infections (e.g., histoplasmosis) and infections due to other opportunistic pathogens. If these develop, discontinue the drug.
- Before starting treatment, perform a test for latent tuberculosis; if positive, start treatment for TB prior to starting the drug. Monitor all patients for development of active TB during treatment, even if the initial latent TB test was negative.
- Lymphoma and other malignancies, some fatal, have been reported in children and adolescents treated with TNF inhibitors.
Adverse reactions: The most common adverse reactions (≥5%) are infections, injection-site reactions, headache, abdominal pain and rash.
Drug Class: CLTA4-Ig
Drug: abatacept (Orencia)19
Adverse reactions: The most common adverse reactions (≥10%) are headache, upper respiratory tract infection, nasopharyngitis and nausea.
Drug Class: IL-12/23 inhibitor
Drug: ustekinumab (Stelara)20
Adverse reactions: The most common adverse reactions (≥3%) are arthralgias and nausea.
Drug Class: Janus kinase (JAK) inhibitor
Drug: tofacitinib (Xeljanz)21
Boxed Warning: Serious Infections, Mortality, Malignancy & Thrombosis
- There is an increased risk of serious cardiovascular events, such as heart attack or stroke, cancer, blood clots and death.22
- There is an increased risk of serious infections leading to hospitalization or death, including TB and bacterial, invasive fungal, viral and other opportunistic infections.
- If a serious infection develops, discontinue treatment until the infection is controlled.
- Before starting treatment, perform a test for latent TB; if positive, start treatment for TB prior to starting the drug. Monitor all patients for development of active TB during treatment, even if the initial latent TB test was negative.
- Thrombosis, including pulmonary embolism, deep venous thrombosis and arterial thrombosis, have occurred in patients treated with JAK inhibitors.
- Lymphoma and other malignancies have been observed in tofacitinib-treated patients. Epstein-Barr virus-associated post-transplant lymphoproliferative disorder has been observed at an increased rate in renal transplant patients treated with tofacitinib and concomitant immunosuppressive medications.
Adverse reactions: The most common adverse reactions (≥2%) are upper respiratory tract infection, nasopharyngitis, diarrhea and headache.
Drug Class: IL-17 receptor antagonist23-25
Drug: brodalumab (Siliq; off-label), ixekizumab (Taltz), secukinumab (Cosentyx)
Boxed Warning for Brodalumab (Siliq): Suicidal Ideation & Behavior
- Suicidal ideation and behavior, including completed suicides, have occurred in patients treated with brodalumab.
- Prior to prescribing, weigh potential risks and benefits in patients with a history of depression and/or suicidal ideation or behavior.
- Patients with new or worsening suicidal thoughts and behavior should be referred to a mental health professional.
- Advise patients and caregivers to seek medical attention for manifestations of suicidal ideation or behavior, new onset or worsening depression, anxiety or other mood changes.
- Brodalumab is available only through a restricted program called the SILIQ REMS Program.
Adverse reactions: The most common adverse reactions (≥1%) are infections, nasopharyngitis and headaches.
Drug Class: IL-23 inhibitors26-28
Drug: guselkumab (Tremfya), tildrakizumab-asmn (Ilumya; offlabel), risankizumab-rzaa (Skyrizi; off-label)
Adverse reactions: The most common adverse reactions (≥1%) are upper respiratory infections, headache, fatigue and injectionsite reactions.
Mary Choy, PharmD, BCGP, FASHP, is a medical writer and editor living in New York City. Dr. Choy is director of pharmacy practice at the New York State Council of Health-System Pharmacists. She is also the author of Healthcare Heroes: The Medical Careers Guide.
References
- Mease PJ, Gladman DD, Papp KA, et al. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol. 2013 Nov;69(5):729–735.
- Singh JA, Guyatt G, Ogdie A, et al. 2018 American College of Rheumatology/National Psoriasis Foundation Guideline for the Treatment of Psoriatic Arthritis. Arthritis Rheumatol. 2019 Jan;71(1):5–32.
- The National Psoriasis Foundation. About Psoriatic Arthritis. 2021 Jun 18. https://www.psoriasis.org/about-psoriatic-arthritis
- The Arthritis Foundation. Using Treat-to-Target for PsA. https://www.arthritis.org/diseases/more-about/using-treat-to-target-for-psa
- U.S. Food & Drug Administration. Otezla prescribing information. 2017 Jun. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205437s006lbl.pdf
- U.S. Food & Drug Administration. Enbrel prescribing information. 2020 Aug. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/103795s5582lbl.pdf
- U.S. Food & Drug Administration. Erelzi prescribing information. 2020 Jun. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761042s012lbl.pdf
- U.S. Food & Drug Administration. Remicade prescribing information. 2020 May. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/103772s5389s5391s5394lbl.pdf
- U.S. Food & Drug Administration. Inflectra prescribing information. 2021 Jun. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125544s018lbl.pdf
- U.S. Food & Drug Administration. Renflexis prescribing information. 2019 Mar. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761054s004lbl.pdf
- U.S. Food & Drug Administration. Ixifi prescribing information. 2020 Jan. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761072s006lbl.pdf
- U.S. Food & Drug Administration. Humira prescribing information. 2021 Feb. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125057s417lbl.pdf
- U.S. Food & Drug Administration. Amjevita prescribing information. 2019 Jun. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761024s004lbl.pdf
- U.S. Food & Drug Administration. Cyltezo prescribing information. 2019 Sep. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761058s003lbl.pdf
- U.S. Food & Drug Administration. Hyrimoz prescribing information. 2021 Feb. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761071s001lbl.pdf
- U.S. Food & Drug Administration. Cimzia prescribing information. 2019 Feb. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125160s289lbl.pdf
- U.S. Food & Drug Administration. Simponi prescribing information. 2019 Sep. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125289s146lbl.pdf
- U.S. Food & Drug Administration. Simponi Aria prescribing information. 2021 Feb. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125433s032lbl.pdf
- U.S. Food & Drug Administration. Orencia prescribing information. 2020 Jun. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125118s194,s199,s225lbl.pdf
- U.S. Food & Drug Administration. Stelara prescribing information. 2020 Dec. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125261s154,761044s006lbl.pdf
- U.S. Food & Drug Administration. Xeljanz prescribing information. 2020 Sep. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213082s000lbl.pdf
- FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. U.S. Food & Drug Administration. Drug Safety Communication. 2021 Sep 1. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-aboutincreased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- U.S. Food & Drug Administration. Siliq prescribing information. 2017 Feb. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf
- U.S. Food & Drug Administration. Taltz prescribing information. 2021 Mar. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125521s014lbl.pdf
- U.S. Food & Drug Administration. Cosentyx prescribing information. 2021 May. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125504s043lbl.pdf
- U.S. Food & Drug Administration. Tremfya prescribing information. 2020 Jul. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761061s007lbl.pdf
- U.S. Food & Drug Administration. Ilumya prescribing information. 2018 Mar. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761067s000lbl.pdf
- U.S. Food & Drug Administration. Skyrizi prescribing information. 2021 Apr. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761105s009s010lbl.pdf