It’s important to select the best therapeutic option for & with the patient to improve adherence & for the patient to attain the highest level of satisfaction.
The Drugs
 Abatacept (Orencia):8 injection/infusion
Abatacept (Orencia):8 injection/infusion
Drug class: selective T cell co-stimulation modulator, DMARD, immunomodulator
Warnings & Precautions
- Concomitant use with a tumor necrosis factor inhibitor (TNFi) can increase the risk of (serious) infections. Discontinue abatacept if a serious infection occurs.
- Anaphylaxis or anaphylactoid reactions can occur after the first infusion and can be life threatening. Appropriate medical support should be immediately available in the event of a reaction. Postmarketing experience reveals at least one case of fatal anaphylaxis following the first abatacept infusion. Following an anaphylactic or other serious allergic reaction, abatacept administration should be stopped immediately with appropriate therapy instituted. Abatacept should be permanently discontinued.
- Patients with a history of recurrent infections or underlying conditions predisposing them to infections may experience more infections.
- Screen for latent tuberculosis (TB) prior to initiating therapy. Patients testing positive should be treated prior to abatacept initiation.
- Live vaccines should not be given concurrently or within three months of abatacept discontinuation.
- Based on its mechanism of action, abatacept may blunt the effectiveness of some immunizations.
- Chronic obstructive pulmonary disease patients may develop more frequent respiratory adverse events.
Dosage & Administration
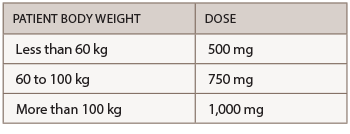
Abatacept is administered by subcutaneous (SQ) injection and intravenous (IV) infusion. The recommended frequency for SQ injection is once weekly, with or without an IV loading dose. For patients initiating therapy with an IV loading dose, administer a single IV infusion (per body weight categories provided in the table below), followed by the first 125 mg SQ injection given within a day of the IV infusion. Patients transitioning from IV therapy to SQ administration should receive the first SQ dose instead of the next scheduled IV dose.
The recommended dose for IV infusion is based on the patient’s body weight.
Commentary
The U.S. Food and Drug Administration (FDA) approved abatacept based on the results of two randomized, controlled trials that involved nearly 3,000 patients. The trials showed that abatacept inhibited joint damage progression independent of patients’ baseline clinical, biochemical or radiographic characteristics. The most common adverse reactions (≥10%) are headache, upper respiratory tract infection, nasopharyngitis and nausea.
Adalimumab (Humira):9 injection
Biosimilars: adalimumab-atto (Amjevita),10 adalimumab-adbm (Cyltezo),11 adalimumab-
adaz (Hyrimoz)12
Drug class: DMARD, TNFi
Boxed warning: Refer to important safety information (ISI) below, and
- Fatal hepatosplenic T cell lymphomas (HSTCLs) have occurred post-marketing in adolescent and young adult inflammatory bowel disease (IBD) patients treated with TNFi’s.
*Important Safety Information
This ISI is applicable to all tumor necrosis factor inhibitors (TNFi’s) and some other immune modulators.
Serious Infections & Malignancies



