Patients with active psoriatic arthritis (PsA) treated with secukinumab show improvements in all disease manifestations and quality-of-life domains established by the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and Outcome Measures in Rheumatology (GRAPPA-OMERACT).1 These findings came from a study by Orbai et al., which examined how PsA patients respond to secukinumab.
Patients with active psoriatic arthritis (PsA) treated with secukinumab show improvements in all disease manifestations and quality-of-life domains established by the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and Outcome Measures in Rheumatology (GRAPPA-OMERACT).1 These findings came from a study by Orbai et al., which examined how PsA patients respond to secukinumab.
Previous randomized studies showed the safety and efficacy of secukinumab for patients with PsA, but all used the traditional measure of efficacy based solely on the ACR response criteria. In this study, investigators pooled this evidence in a post hoc analysis to gather more information on the efficacy of secukinumab in specific, individual disease activity core domains for PsA, including musculoskeletal disease activity (i.e., arthritis, enthesitis, dactylitis and spine symptoms), skin disease activity (i.e., psoriasis and nail psoriasis), pain, patient global assessment, physical function, fatigue, health-related quality of life and systemic inflammation.
“Instead of reporting on ACR20 as the efficacy outcome reported in the primary analysis of each individual trial, we looked individually at those dimensions of PsA representing disease resolution,” says lead author Ana-Maria Orbai, MD, MHS, assistant professor of medicine and director, Psoriatic Arthritis Program, Johns Hopkins Arthritis Center, Division of Rheumatology, Johns Hopkins University School of Medicine, Baltimore.
Post hoc Analysis of Pooled Data
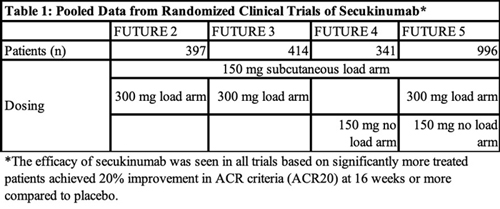
In the study, Orbai et al. pooled data from four on-label phase 3 clinical trials—FUTURE 2, 3, 4 and 5—that demonstrated the efficacy of secukinumab for PsA at approved doses (see Table 1, below). All four of the trials had a loading dose arm of 150 mg of subcutaneous secukinumab, while three of the trials (FUTURE 2, 3 and 5) included a loading dose arm of 300 mg of subcutaneous secukinumab and two trials (FUTURE 4 and 5) had a no-loading dose arm in which patients received 150 mg of secukinumab. Patients were pooled together from these trials based on treatment dose and delivery.
Two thousand forty-nine patients were included in the post hoc analysis. Of these, 461 patients received 300 mg of secukinumab and 572 received 150 mg of secukinumab (each group received treatment at weeks 1, 2, 3 and 4, followed by treatment every four weeks after the initial baseline loading dose). Three hundred and thirty-five received 150 mg of secukinumab every four weeks without a baseline loading dose. A total of 681 patients received placebo. All patients were treated for 16 weeks.