A Closer Look at Development of the MDHAQ/RAPID3
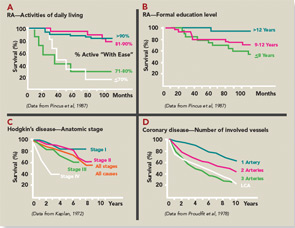
Evolution of a two-sided, one-page MDHAQ continued as one of the few questionnaires developed in actual clinical (rather than research) settings to improve care (see Table 1)—although much research data emerged.6 In 1995, it was recognized that improved patient status led to “floor effects” on the HAQ and MHAQ, i.e., scores of zero although patients nonetheless had functional limitations. Therefore, queries concerning complex activities were added—initially six and ultimately two: “walk two miles or three kilometers” and “participate in sports and recreation as you would like” (see Figure 1).7,8 Queries concerning sleep, anxiety, and depression were added, scores of which are as high or higher than for most traditional HAQ items.8 Visual analog scales (VAS) for pain and global status were converted from a 10-cm line to 21 circles (in increments of 0.5) (see Figure 1), so that a ruler is no longer required for scoring.12
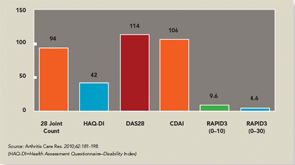
Scoring templates were developed for Routine Assessment of Patient Index Data (RAPID)3, a simple 0–30 composite of three 0–10 scores for physical function, pain and patient global estimate, the 3 RA Core Data Set measures.13,14 A self-report joint count was added from the Rheumatoid Arthritis Disease Activity Index (RADAI) questionnaire.15 RADAI and the psychological status queries were scored formally in earlier versions, as RAPID4 and RAPID5, but RAPID3 scores gave similar information in much less time.13 Therefore, formal scoring of these scales is no longer advocated, though the scales provide highly useful clinical information.
The second page of the MDHAQ (see Figure 2) includes a review of systems and recent medical history. More than 25 checks for positive symptoms is characteristic of patients with fibromyalgia, some of whom may also meet criteria for diseases such as RA or SLE; this information proves quite helpful as a clue that symptoms may be due to fibromyalgia rather than inflammation, a difficult determination in some patients.16 A recent medical history summary can save at least two to three minutes for the doctor if all answers are “no,” whereas any “yes” responses should be known.17 Queries concerning change in status, exercise, a visual analog scale for fatigue, demographic measures, consent for further monitoring, and contact in the future should the patient change clinical settings also have proven their value over and over.