NIH investigators describe key features and genetic causes of Mendelian interferonopathies, & treatment approaches that may indicate the efficacy of IFN inhibition.


NIH investigators describe key features and genetic causes of Mendelian interferonopathies, & treatment approaches that may indicate the efficacy of IFN inhibition.
Bradley Bohman, MD, & Jawad Bilal, MBBS |
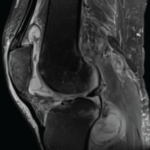
Tumor necrosis factor-α inhibitors (TNFi’s) have emerged as an integral part of therapeutic strategies for several rheumatic diseases. TNF-α is a pro-inflammatory cytokine implicated in the pathogenesis of rheumatoid arthritis (RA), seronegative spondyloarthropathies and inflammatory bowel disease (IBD). It also plays a central role in the immune response to mycobacterial infection. Many biologic agents, particularly…

Three FDA representatives discuss new drug indications, safety precautions and label changes, & an emergency program to rapidly evaluate existing immunomodulating therapies for use in COVID-19 patients.

Elizabeth (Blair) Solow, MD |
ACR CONVERGENCE 2020—Held Nov. 5–9, the ACR’s first fully virtual annual meeting provided participants with a vast repository of new research related to rheumatoid arthritis (RA). To help you sort through the noise, Elizabeth (Blair) Solow, MD, an assistant professor of medicine in the Division of Rheumatic Diseases at UT Southwestern Medical Center, Dallas, offered …

ACR CONVERGENCE 2020—In many ways, the current plethora of treatment options for rheumatoid arthritis patients represents an embarrassment of riches. However, while many therapeutics approved by the U.S. Food & Drug Administration (FDA) are available, knowing the order in which to try these medications with patients can be quite challenging. In The Great Debate, held…

Larry Beresford |
An observational study found treatment with tofacitinib resulted in only a slightly higher rate of venous thromboembolism than tumor necrosis factor inhibitors in patients with rheumatoid arthritis.

During the 2020 ACR State-of-the-Art Clinical Symposium, Zoltán Szekanecz, MD, PhD, addressed the risks of vascular disease and how to manage them in patients with rheumatic diseases.

Kimberly Retzlaff |
SNOWMASS VILLAGE, COLO.—Current trends in rheumatoid arthritis (RA) therapy are the increased use of newer medication categories, such as Janus kinase (JAK) inhibitors (Jakinibs) and biologics, and the rising costs of treatment. Unchanged is the consistent use of methotrexate as an effective therapy. These topics and more were discussed at the ACR Winter Symposium during…

Kimberly Retzlaff |
SNOWMASS VILLAGE, COLO.—Janus kinase inhibitors—or Jakinibs—are a relatively new class of disease-modifying anti-rheumatic drugs (DMARDs) that perform well and have a safety profile comparable to biologics. This group of drugs was the subject of The New Frontier: Comparative Safety of JAK Inhibitors, a presentation given at the ACR Winter Symposium by Kevin L. Winthrop, MD,…
Vania Lin, MD, MPH, & Leah Krull, MD |
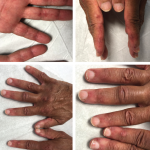
Clinically amyotrophic dermatomyositis (CADM), a subset of dermatomyositis (DM), is a rare autoimmune disease characterized by typical DM cutaneous findings (e.g., heliotrope rash, Gottron papules, Gottron sign) without evidence of myositis.1 The incidence of DM and CADM is approximately 9.63 per 1 million people and 2.08 per 1 million people, respectively.2 The association with development…