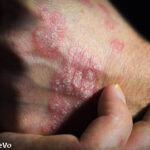
It seems to have begun in Norway, the international pressure to switch patients from well-known brand biologic agents, such as Remicade (infliximab), to biosimilar agents, due to a significant cost advantage.1 This biosimilar came with a 39% price markdown last year compared with the brand, and when Norway called for bids, the discount went to…