NEW YORK (Reuters Health)—People with persistent pain need better access to psychosocial care, according to a position statement from the Society of Behavioral Medicine (SBM). “Psychosocial approaches to pain management need to be available for all individuals with persistent pain in all healthcare settings,” Dr. E. Amy Janke from the University of the Sciences, in…
Search results for: opioid

FDA Receives Reports of Loperamide Abuse
The FDA is seeking to limit the number of loperamide doses per package due to reports of heart-related problems and death from the misuse and abuse of the treatment…

Upadacitinib Receives Breakthrough Designation, Abatacept Use Expands in Australia & More
The FDA has designated upadacitinib a breakthrough therapy to treat adults with moderate to severe atopic dermatitis…
U.S. Senate Votes to Confirm Azar as Health Secretary
WASHINGTON (Reuters)—The U.S. Senate on Wednesday voted to confirm former pharmaceutical industry executive and lobbyist Alex Azar as the next Health and Human Services secretary. Azar will oversee the Trump administration’s response to the opioid epidemic, its efforts to weaken the Affordable Care Act, commonly called Obamacare, and address rising prescription drug prices. The Senate…

2017 ACR/ARHP Honors & Awards, Part 2
SAN DIEGO—At the 2017 ACR/ARHP Annual Meeting in San Diego in November, the ACR and the ARHP honored a group of distinguished individuals who have made significant contributions to rheumatology research, education and patient care. This month, The Rheumatologist speaks with the ARHP winners about their individual contributions. In addition, we profile the new class…
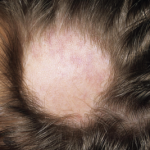
Tips for Treating Lupus-Related Renal Disease, Pain, Alopecia
SAN DIEGO—Rheumatologists who treat lupus patients gleaned tips on diagnosis and management of renal disease, painful neuropathies and alopecia at a “Curbside Consults” session held Nov. 6 at the ACR/ARHP Annual Meeting in San Diego. Membranous Lupus Nephritis Patients with refractory membranous lupus nephritis (MLN), or Class V lupus nephritis, face “significant morbidity, most of…
U.S. Senate Committee Advances Azar Nomination as Health Secretary
WASHINGTON (Reuters)—A U.S. Senate committee on Wednesday voted to move forward the nomination of Alex Azar, a former drug industry executive and lobbyist whom President Donald Trump has tapped to be the next secretary of Health and Human Services. The Senate Finance Committee voted 15-12 to advance Azar’s nomination, with all but one Democrat opposing….
Health Secretary Nominee Indicates Support for Medicaid Overhaul
WASHINGTON (Reuters)—Alex Azar, a former drug industry executive and lobbyist nominated to run the U.S. Department of Health and Human Services, indicated on Tuesday he supported a Republican bid to overhaul Medicaid and again vowed to tackle high drug prices. Azar appeared before the Senate Finance Committee on Tuesday, which will ultimately decide whether to…

Unwise Choices: EHRs, PBMs, Drug Costs Are Leading to Physician Burnout
My dear electronic health records How do I dislike thee? Let me count the ways Adaptation of Sonnet 43 By Elizabeth Barrett Browning, 1806–1861 As my tenure as physician editor winds down, it’s worth reviewing some of the more nettlesome issues confronting clinicians that have been previously discussed in these pages and gauge their current…

Pain Treatments Move Closer to U.S. Market
Two pain treatments, extended-release injectable suspension triamcinolone acetonide (Zilretta) and meloxicam, have seen movement at the U.S. Food and Drug Administration (FDA). In October, the agency approved Zilretta to treat osteoarthritis (OA) knee pain and accepted a new drug application for meloxicam to treat pain. FDA Approves Zilretta On Oct. 6, the FDA approved extended-release,…
- « Previous Page
- 1
- …
- 17
- 18
- 19
- 20
- 21
- …
- 32
- Next Page »