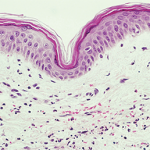
WASHINGTON, D.C.—Often, no clear explanation exists in neutrophilic dermatosis cases that links a patient’s skin disorder with an internal condition, expert Joseph Jorizzo, MD, professor, founder and former chair of the Dermatology Department at Wake Forest University in Winston-Salem, N.C., and professor of clinical dermatology at Wevascularill Cornell Medical College in New York, told attendees…