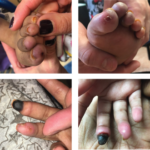
A key question many graduating rheumatology fellows face each year is: Are you interested in pursuing a career in academic medicine or in private practice? Although the two tracks are not mutually exclusive, it is true that juggling the demands of scholarly work, medical education and a busy clinical workload is by no means easy….