RANKL as well as M-CSF, the key mediators of osteoclastogenesis, show prominent expression in the synovium of patients with RA; this expression, however, does not appear to be balanced by the expression of regulatory molecules such as RANKL’s decoy receptor osteoprotegerin.2 In addition, inflammatory cytokines that induce RANKL, such as IL-1, IL-6, and TNF, are abundantly expressed in the synovial tissue of RA.4 The strong expression of RANKL in inflamed RA joints makes this molecule a potential important target for novel antiresorptive therapy. Evidence of this possibility comes from studies showing that RANKL-deficient mice are resistant to inflammation-induced bone loss but nevertheless show similar level of inflammation as their wild-type littermates.2,4
In humans, blockade of RANKL with the humanized monoclonal antibody denosumab is effective not only in the treatment of postmenopausal osteoporosis and bone loss from cancer metastasis but also in inhibiting bone destruction in RA.17–19 The strong effects of denosumab on erosion in RA are remarkable because classic antiresorptive drugs such as bisphosphonates have only minor effects on bone destruction in RA joints.4 Standard anticytokine therapies in RA such as the blockade of TNF-α and IL-6R may also influence bone loss in RA by acting on the RANKL–RANK system.2,4 In this context, the link between pro-inflammatory cytokines and osteoclast formation may explain why anticytokine therapy is effective in inhibiting structural damage in RA.
Periarticular Bone Loss in RA
In addition to bone erosions at the joint margins, patients with RA also may display periarticular bone loss in the trabecular bone in the vicinity of the inflamed joints. The presence of these lesions could indicate that the bone marrow is part of the disease process in arthritis. MRI studies in patients with RA support this notion because they reveal a high prevalence of bone marrow lesions in RA. Histological analysis of such lesions reveals (water-rich) vascularized inflammatory infiltrates, which replace the bone marrow fat and contain aggregates of B and T cells. Of note, similar if not identical MRI changes appear early in the disease process of RA and may predict joint damage in RA.2,7
New Bone Formation in SpA
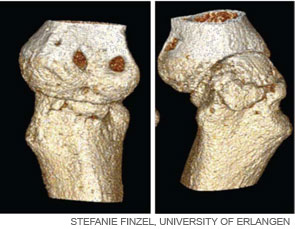
In RA, there are few signs of repair of bone erosions. At first glance, the paucity of repair is striking because bone formation is usually linked with bone resorption. Thus, increased rates of bone resorption should lead to increased bone formation.7 When compared with RA, the spondyloarthropathies display important differences in the pattern and pathophysiology of joint disease. SpA frequently affects amphiarthroses of the axial skeleton, which lack a true joint cavity and a synovial lining tissue. In addition, entheses, which are sites of tendinous or ligamentous attachments to bone, typically represent the initial sites of inflammation in SpA. In strong contrast to bone erosion in RA, however, bone abnormalities in the SpA are dominated by new bone formation.