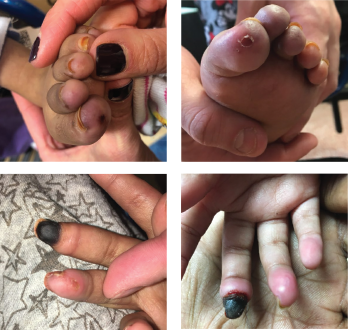
Figures 1A-D: A 5-year-old girl presented with erythema and ulcerations of the finger- and toetips, which had progressed to ischemia of the right, third fingertip.
Janus kinase 1 and 2 inhibitors (jakinibs) have been approved for the treatment of rheumatoid arthritis, psoriatic arthritis and, most recently, juvenile idiopathic arthritis. They have also shown promise in the treatment of interferon (IFN) mediated diseases. The Janus kinase and signal transducer and activator of transcription (JAK/STAT) pathway is the principal signaling pathway for IFN receptors, which are upregulated in IFN-mediated diseases, such as Aicardi-Goutières syndrome.
Here, we present a case of a child who presented with a necrotic digit and was found to have Aicardi-Goutières syndrome, which had previously been misdiagnosed as metachromatic leukodystrophy. She would potentially have benefited from treatment with a jakinib, but was unable to obtain insurance approval for this medication.
Case Presentation
A 5-year-old girl with a diagnosis of metachromatic leukodystrophy, spastic quadriplegia and developmental delay presented with a six-week history of erythematous discoloration and ulcerations of the fingertips and toes (see Figures 1A–B) and ischemia of her right third fingertip (see Figures 1C–D). She was admitted to the hospital for evaluation and treatment of the ischemic digit.
She had no history of fevers or joint swelling, no known trauma and no significant cold exposure to the affected areas. In addition to the findings described on her digits, a physical exam revealed normal vital signs, hypertonia and contractures consistent with her metachromatic leukodystrophy diagnosis, no swollen joints or effusions, and no other skin lesions or rashes. Her respiratory and cardiac exams were also unremarkable.
An evaluation targeting a diagnosis for her ischemic finger was undertaken. The investigation for an underlying hypercoagulable state was negative. She had a prothrombin time test, which revealed a normal international normalized ratio (INR), and her fibrinogen, protein C and S, and antithrombin 3 levels were within normal ranges. Tests for antiphospholipid antibodies and cryoglobulins were negative.
An echocardiogram was obtained to assess for a thromboembolic etiology and did not demonstrate valvular vegetations.
A vasculitis or underlying autoimmune disease with associated vasospasm was considered. A computed tomography (CT) scan of her head, neck, chest, abdomen and pelvis revealed normal vasculature.
Her erythrocyte sedimentation rate (ESR) was 95 mm/hr (reference range [RR] 0–15 mm/hr). Her total IgG was elevated at >2,000 mg/dL (RR: 454–1,360 mg/dL). She had an unremarkable urinalysis and her serum creatinine, C3 and C4 were all within the normal range. Her anti-nuclear antibody (ANA), double-
stranded DNA and anti-neutrophil cytoplasmic antibody (ANCA) were all negative. An extractable nuclear antigen (ENA) antibody panel was also negative.
Two skin biopsies were performed. The first biopsy showed some dermal mucin deposition, which can be seen in chilblain lupus erythematosus, but a repeat biopsy was nonspecific, demonstrating only perivascular inflammation.
At this point, she received a diagnosis of perniosis without any clear underlying autoimmune disease. Her care team took a step back, and in the setting of her neurologic abnormalities, they considered Aicardi-Goutières syndrome as a diagnosis that could elegantly tie together her acute presentation with her past history of spastic quadriplegia and developmental delay.
We undo much of the promise of new treatment options when the patients who could most benefit from them cannot receive them.
Aicardi-Goutières syndrome is a genetically heterogeneous disorder that is part of a group of diseases characterized by excessive interferon signaling. In Aicardi-Goutières syndrome, neurologic insults, such as intracranial calcification, white matter changes and cerebral atrophy, are common, and can result in developmental delay and contractures, as observed in our patient.
Another feature of Aicardi-Goutières syndrome, observed in about 40% of cases and more commonly seen in those with the SAMHD1 mutation, is perniosis or chilblains, as seen in our patient.5,8 This is one of the most distinctive extra-neurological manifestations of Aicardi-Goutières syndrome and highlights the overlap of Aicardi-Goutières syndrome with some of the pathophysiology seen in systemic lupus erythematosus, such as disturbance of IFNα metabolism.1,3
Failure of primer repair exonucleases has been presumed to result in intracellular DNA/RNA, which triggers an inappropriate immune response and overproduction of IFNα.3,8 This overproduction can result in an IFN-induced vasculitis, manifesting as skin lesions and leukodystrophy, which can be seen in Aicardi-Goutières syndrome.
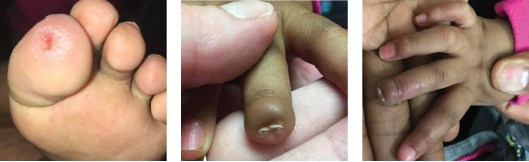
Figure 2: The patient’s erythema and ulcerations improved, and her ischemic digit auto-amputated.
With this in mind, the team revisited the patient’s diagnosis of metachromatic leukodystrophy. Further review of her history revealed that she had a normal birth and was initially developing normally. However, when she did not crawl or stand up when expected, she was referred to a neurologist and brain imaging was performed, which resulted in the diagnosis of metachromatic leukodystrophy.
Magnetic resonance imaging (MRI) of the brain was repeated and showed periventricular, white matter abnormalities consistent with metachromatic leukodystrophy, but also consistent with Aicardi-Goutières syndrome.
The patient’s arylsulfatase A level was found to be within normal ranges, providing strong evidence against a diagnosis of metachromatic leukodystrophy. Genetic testing revealed a homozygous mutation in the SAMHD1 gene—one of the gene mutations identified in patients with Aicardi-Goutières syndrome.
Upon further review of the family history, it was discovered the parents were second cousins. Genetic testing of her siblings found four of six had heterozygous mutations, but were otherwise healthy. (Note: Heterozygous individuals can also have clinical manifestations of this disease, so her siblings could now be monitored and receive interventions as appropriate.)
Our patient was treated with systemic glucocorticoids, azathioprine, aspirin, amlodipine and topical treatments for the perniosis. Her erythema and ulcerations improved (see Figure 2A). Eventually, her fingertip auto-amputated (see Figures 2B–C), and she did not have initial recurrence of her perniosis.
The family’s language barrier, low health literacy and obligations of caring for six other children made treatment adherence difficult, especially when it came to following a steroid taper. Given our patient’s risk of eventual relapse and in an effort to simplify her medical regimen while improving her overall outcome, we attempted to start a JAK 1/2 inhibitor (jakinib), given the promising data surrounding this class of medication for the treatment of interferonopathies.2,4,6,7
In one case study of an adult patient who also had perniosis in the setting of Aicardi-Goutières syndrome, the patient achieved complete remission with baricitinib.4 A recent study published in the New England Journal of Medicine evaluated 35 patients with genetically confirmed Aicardi-Goutières syndrome, and compared their interferon signaling gene expression score, symptoms associated with their disease and symptom development from before the patients started treatment with baricitinib with scores and symptoms found during treatment. The researchers found that interferon signaling gene expression scores decreased during treatment, as did reported symptoms by parents during treatment. Perhaps most important, many more children gained developmental milestones during treatment than before, and the number of milestones gained seemed to correlate with a higher dose of baricitinib.7
Unfortunately, our patient’s insurance repeatedly denied baricitinib or other jakinibs, despite our strong petitioning and legal hearing. At the time of this report, she was lost to follow-up. The stress from having to involve the legal system in attempts to get this medication approved may have contributed to this loss.
Discussion
This case reminds us to consider interferon-mediated diseases when presented with patients with vasculopathies and neurologic dysfunction. The success of correcting this patient’s diagnosis came from the team’s ability to take a step back and question what was already felt to be known—an important part of what rheumatologists do every day. Given the rarity of these diseases, patients with interferon-mediated diseases may often be misdiagnosed. Having a high index of suspicion when we see the combination of vasculopathy and neurologic dysfunction can avoid a delay in treatment, which can potentially prevent permanent, neurologically devastating effects of these diseases.
This case also considers the exciting promise of jakinibs in the treatment of an increasing number of autoimmune diseases, as well as the ongoing need for more research into this class of medications to explore their benefits, elucidate optimal doses and characterize side effects, among other factors.
Lastly, this case also serves as an all too common example of how social determinants of health can magnify disease burden and also serves as a reminder that addressing access to care, coverage of new therapies and culturally appropriate care are just as important as investing in exciting new pharmaceuticals. We undo much of the promise of new treatment options when the patients who could most benefit from them cannot receive them.
Marta Michalska-Smith, MD, is a first-year rheumatology fellow at The George Washington University, Washington, D.C. She completed her residency training in Medicine-Pediatrics at the University of Minnesota.
Colleen K. Correll, MD, MPH, is an assistant professor in the Division of Pediatric Rheumatology at the University of Minnesota. Her area of research focus is in epidemiology of juvenile idiopathic arthritis.
References
- Abdel-Salam GMH, El-Kamah GY, Rice GI, et al. Chilblains as a diagnostic sign of Aicardi-Goutières syndrome. Neuropediatrics. 2010;41(1):18–23.
- Fragoulis GE, McInnes IB, Siebert S. JAK-inhibitors. New players in the field of immune-mediated diseases, beyond rheumatoid arthritis. Rheumatology. 2019;58(Suppl 1):i43–i54.
- Kolivras A, Aeby A, Crow YJ, et al. Cutaneous histopathological findings of Aicardi-Goutières syndrome, overlap with chilblain lupus. J Cutan Pathol. 2008 Aug;35(8):774–778.
- Meesilpavikkai K, Dik WA, Schrijver B, et al. Efficacy of baricitinib in the treatment of chilblains associated with Aicardi‐Goutières syndrome, a type I interferonopathy. Arthritis Rheumatol. 2019 May;71(5):829–831.
- Prendiville JS, Crow YJ. Blue (or purple) toes: Chilblains or chilblain lupus-like lesions are a manifestation of Aicardi–Goutières syndrome and familial chilblain lupus. J Am Acad Dermatol. 2009;61(4):727–728.
- ontealegre Sanchez GA, Reinhardt A, Ramsey S, et al. JAK1/2 inhibition with baricitinib in the treatment of autoinflammatory interferonopathies. J Clin Invest. 2018 Jul 2;128(7):3041–3052.
- Vanderver A, Adang L, Gavazzi F, et al. Janus kinase inhibition in the Aicardi–Goutières syndrome. N Engl J Med. 2020 Sep 3;383(10):986–989.
- Yarbrough K, Danko C, Krol A, et al. The importance of chilblains as a diagnostic clue for mild Aicardi-Goutières syndrome. Am J Med Genet A. 2016 Dec;170(12):3308–3312.


