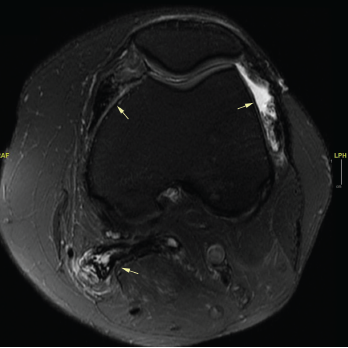
Figure 1. An MRI of the patient’s left knee with and without IV contrast. The knee featured diffuse pigmented villonodular synovitis, including within a lobulated Baker’s cyst, without substantial change in disease burden dating back to 2017.
Her tumor was stable off therapy as demonstrated by magnetic resonance imaging (MRI) of her left knee, which identified diffuse pigmented villonodular synovitis and a lobulated Baker’s cyst without substantial change in disease burden compared to one year ago (see Figure 1).
On physical exam, the patient had normal vital signs and was not in acute distress. She had normal heart and lung sounds, and a soft abdomen that was not tender on palpation. She had a post-inflammatory, hyperpigmented rash on her extremities, but no active lesions. Both shoulders and biceps were diffusely tender to palpation, and her left knee had a moderate joint effusion and tenderness to palpation with an inability to fully extend.
Strength testing revealed 4-/5 muscle strength in neck flexion and extension, 4/5 muscle strength in the proximal upper extremities, 4-/5 muscle strength in bilateral hand grip, 4-/5 muscle strength in bilateral hip flexion, 4/5 muscle strength in knee extension, 4/5 muscle strength in ankle dorsiflexion and 4/5 muscle strength in bilateral toe flexion and extension.
Her laboratory tests during the clinic visit appear in Table 1.
She had no history of neck irradiation or thyroid surgery. One year prior to presentation, she had normal thyroid function tests, but had elevated thyroid peroxidase (TPO) antibodies at 585 mIU/L (RR: ≤5.61 IU/mL) and anti-thyroglobulin antibodies at 77.96 IU/mL (RR: ≤4.11 IU/mL); and normal thyroid-stimulating immunoglobulin at 99% (RR: ≤122%). She developed transient thyroiditis, with palpitations, tremors and low TSH two months after starting durvalumab, and developed hypothyroidism one month after stopping durvalumab. The patient was compliant with levothyroxine (Synthroid) until stopping the medication five months after stopping durvalumab because she misunderstood the instructions.
An MRI of both thighs, with and without contrast, revealed mild diffuse muscle edema in the adductor magnus and semimembranosus muscles bilaterally, worse on the left side (see Figure 2). A muscle biopsy of her left quadriceps on pathological review identified scattered atrophic fibers largely, but not completely, restricted to type 2; there was no evidence of myonecrosis, regeneration or inflammation, and no evidence of significant immunohistochemical upregulation of major histocompatibility complex (MHC) class I and II (see Figure 3).
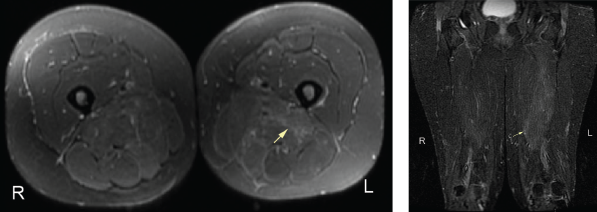
Figure 2. An MRI of the patient’s left and right femur without IV contrast. Note the mild diffuse muscle edema in the adductor magnus and semimembranosus muscles bilaterally, the left greater than right.
The myositis panel was canceled due to an inadequate blood sample.
Differential diagnoses in this case included paraneoplastic myositis secondary to underlying malignancy, inflammatory polymyositis or dermatomyositis as an IRAE from the PD-1L inhibitor, elevated CK as a side effect of the CSF-1R inhibitor, polymyositis associated with infliximab treatment, and hypothyroid-related myopathy as an IRAE from the PD-1L inhibitor. Inflammatory myositis was unlikely given the unremarkable muscle biopsy and normal inflammatory markers.



