Immune checkpoint inhibitors (ICIs) are at the forefront of advances in cancer therapy and have shown promising results for progression-free survival. Checkpoint signaling pathways, such as cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1), normally regulate the immune response to promote self-tolerance and prevent tissue damage and inflammation.
PD-1 is a co-inhibitory receptor expressed on activated T cells. It “turns off” T cells that would normally destroy cancer cells. Solid tumors have increased expression of PD-1, which is often associated with a worse prognosis. Blockage of PD-1 with ICIs enhances T cell function and tumor lysis. This is the basis of using monoclonal antibodies to PD-1 inhibitors as targeted immunotherapy cancer agents.1,2
PD-1 inhibition not only enhances the immune response to tumor cells but also to normal host tissue. Immune-related adverse events (irAEs) from ICIs occur from self-intolerance with inhibition of checkpoint sites and uncontrolled activity of T cells, leading to a multi-organ inflammatory response that can mimic a rheumatologic condition. irAEs, graded 1–4, can occur in variable frequency at any time during therapy. They have been reported in higher numbers with the use of CTLA4 inhibitors, such as ipilimumab, or combination PD1 inhibitors (e.g., nivolumab, pemrolizumab) but less commonly with nivolumab monotherapy.2,3 Nivolumab (Opdivo) has been approved for melanoma, squamous cell cancer of the head and neck, Hodgkin’s lymphoma, colorectal, renal cell and non-small cell lung cancer.
irAEs can involve any organ system, but myalgias, arthralgias, gastrointestinal, dermatologic, hepatic and endocrine autoimmune effects are those most commonly reported with nivolumab use. Cardiac, pulmonary and ocular toxicities are rare.3-5
Nivolumab and ICIs are being tested for other malignancies, such as ovarian cancer, which is the most lethal of gynecologic malignancies, with a five-year survival rate of 25% for women diagnosed with advanced stage disease despite surgery and chemotherapy. Recurrence is associated with poor prognosis and resistance to standard treatment. Recent research in oncology has shown that ovarian cancers have an increased expression of PD-1 and poorer outcomes; PD-1 inhibition is the basis of current investigations for nivolumab’s use in ovarian cancer.6
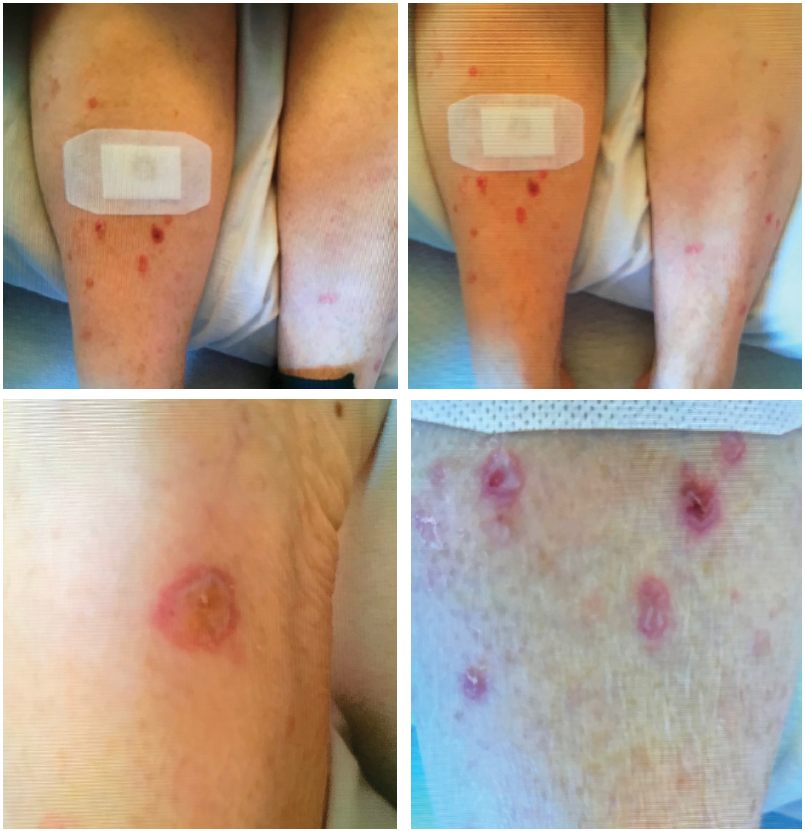
The patient presented with multiple, bullous, round, erythematous plaques on both of her lower, distal extremities. Biopsy showed perivascular inflammatory cell infiltrate concerning for drug eruption, collagen vascular disease or early autoimmune vesiculobullous disorder.
We present the case of a patient with a history of cardiac disease on statin therapy and ovarian cancer treated with nivolumab who developed cardiac, musculoskeletal, respiratory, neurologic, dermatologic and gastrointestinal autoimmune adverse events. This case is unique for several reasons:
- Nivolumab was used as a novel agent for ovarian cancer;
- Its use resulted in multiple autoimmune toxicities, as well as potentially life-threatening manifestations of cardiotoxicity and inflammatory myositis that are rarely reported with its use; and
- Ovarian cancer and statin use itself can lead to autoimmune myositis and needed to be differentiated from nivolumab toxicity.
Case Presentation
A 75-year-old woman with a past medical history of hypertension, osteopenia, coronary artery disease with one stent, hyperlipidemia on 20 mg of atorvastatin and no known personal or family history of rheumatologic disorders was diagnosed with stage 3c, high-grade, serous ovarian cancer in 2014. She was treated with hysterectomy, oophorectomy and multiple cycles of paclitaxel and carboplatin, but the cancer recurred in 2015 with hepatic metastasis. She received further cycles of carboplatin and docetaxel and went into remission.