Unlike historical drug-specific safety registries, which are limited in scope and participation, this format allows a more robust estimation of the risk of rare long-term adverse events, such as malignancies and opportunistic infections in exposed and non-exposed individuals. It also allows improved understanding of long-term outcomes in real-life settings with mechanisms in place to continue follow-up into adulthood even as patients transition out of the pediatric age range.
The FDA endorsed this approach to pharmacosurveillance, and several companies have engaged CARRA to use the Registry to satisfy post-marketing requirements.15 As of summer 2016, the CARRA Registry had enrolled almost 1,500 patients, mostly with polyarticular and systemic JIA. Other pediatric rheumatic diseases will be added as funding allows, beginning with SLE in 2016.
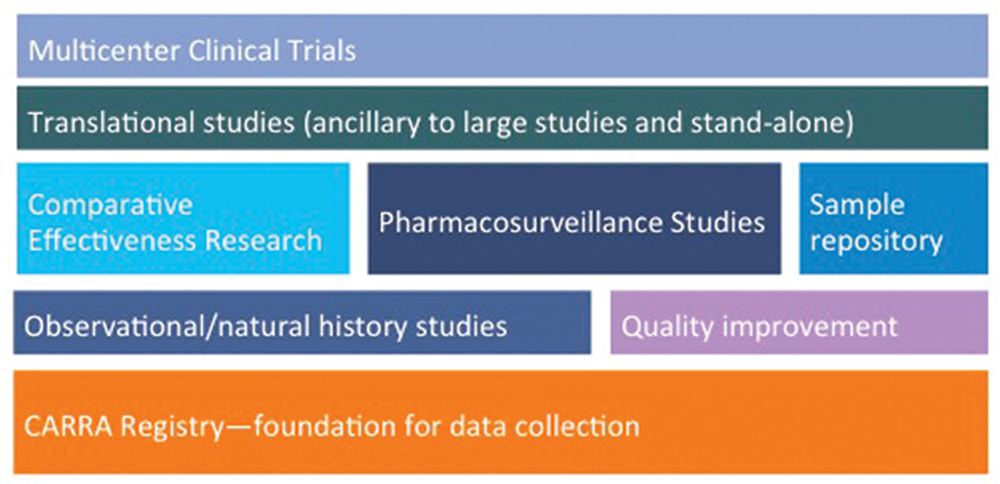
The “new” CARRA Registry has become the preferred data-collection vehicle for all large multicenter CARRA studies, which are not limited to pharmacosurveillance, observational and natural history studies (see Figure 4). Ongoing studies of comparative effectiveness in an observational setting are feasible and economical compared with randomized active comparator trials and provide a research model for studies in other rare diseases. One such example, STOP-JIA (Start Time Optimization of biologics in Poly-JIA), funded by the Patient-Centered Outcomes Research Institute (PCORI), will compare the effectiveness of the polyarticular JIA CTPs to answer the pressing clinical question of when biologics should be started in a patient with new-onset polyarticular JIA.
(click for larger image)
Figure 4: Planning & Building the CARRA Research Portfolio on the Foundation of the CARRA Registry
The breadth of the current and planned research initiatives using the CARRA Registry as the foundation for data collection.
Similarly designed is FROST (First-Line Options for Systemic JIA Treatment), which is due to begin fall 2016 and will compare the CARRA systemic JIA CTPs in children with new-onset systemic JIA.
Because these are observational studies with standard of care treatments, data collection as outlined in the general CARRA Registry protocol and utilize existing site contracts, operationalizing these studies is relatively easy and efficient compared with starting stand-alone studies.
Companion translational studies leveraging the biospecimen infrastructure within the network are being implemented for both STOP-JIA and FROST, focusing on patients before and after starting biologic or DMARD therapy, and aim to identify biomarkers that predict response and non-response, as well as aiding in diagnosis.
Moving forward, translational studies and appropriate biospecimen collection will be a routine part of CARRA studies, creating a robust resource of highly phenotyped samples linked to longitudinal clinical data to fuel other translational and basic investigation. Investigator-initiated and industry-sponsored randomized clinical trials are also in development and will take advantage of the CARRA Registry infrastructure and network.

