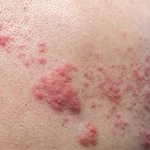
In Summary
This study found that patients with RA who initiated treatment with JAK inhibitors had a very high incidence of herpes zoster, with almost double the risk of patients who initiated treatment with TNF inhibitors. The risk of serious bacterial infections was comparable between the groups; however, the risk of developing an opportunistic infection—tuberculosis in particular—was lower for JAK inhibitor-treated patients than TNF inhibitor-treated patients.
This study did not examine all drug classes and was not randomized, which limits the strength of any recommendations that can be made on these findings. However, it’s important to understand which agents have a higher propensity for infection because these treatments provide remarkable results for controlling RA, limiting long-term disability and improving patient quality of life. Understanding the magnitude of infection risk with different therapies can help clinicians and their patients weigh the risks and benefits of different treatment regimens in shared decision making. These findings also serve as a reminder to clinicians to screen patients for tuberculosis prior to initiating therapy.
Michele B. Kaufman, PharmD, BCGP, is a freelance medical writer based in New York City and a pharmacist at New York Presbyterian Lower Manhattan Hospital.
References
- Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology Guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2021 Jul;73(7):924–939.
- Highlights of prescribing information: Enbrel (etanercept). U.S. Food & Drug Administration. 2022 Aug 16.
- Package insert: Humira (adalimumab). U.S. Food & Drug Administration. 2002 Dec.
- Highlights of prescribing information: Remicade (infliximab). U.S. Food & Drug Administration. 2021 Oct. 5.
- Highlights of prescribing information: Simponi aria (golimumab). U.S. Food & Drug Administration. 2013 July 18.
- Highlights of prescribing information: Cimzia (certolizumab pegol). U.S. Food & Drug Administration. 2006 Apr 22.
- Highlights of prescribing information: Olumiant (baricitinib). U.S. Food & Drug Administration. 2018 May 31.
- Highlights of prescribing information: Xeljanz/ER (tofacitinib). U.S. Food & Drug Administration. 2016 Feb 23.
- Highlights of prescribing information: Rinvoq (upadacitinib). U.S. Food & Drug Administration. 2019 Aug 16.
- Choi S, Shin A, Ha Y, et al. Risk of infections between JAK inhibitors and TNF inhibitors among patients with rheumatoid arthritis [abstract: 0302]. Arthritis Rheumatol. 2022 Oct;74(suppl 9).