In addition to the primary histologic pattern within the interstitium, certain microscopic features suggest the presence of “autoimmune lung disease.” These include dense perivascular collagen, intense plasmacytic infiltration, extensive pleuritis, and numerous lymphoid aggregates with germinal center formation. Whether or exactly how these features affect response to immunomodulatory therapy or prognosis has yet to be definitively determined.
What Does High-Resolution Computed Tomography Tell Us?
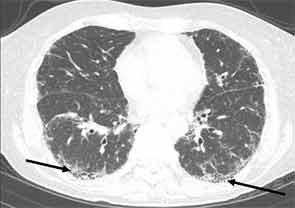
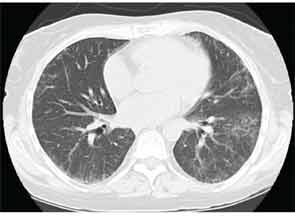
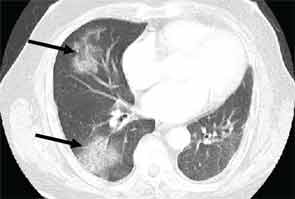
High-resolution computed tomography (HRCT) is the imaging modality of choice to assess ILD and can accurately predict the underlying histological pattern in many cases (see Figures 1–3). For example, when lower zone-, posterior-, subpleural-predominant reticular opacities with traction bronchiectasis and honeycombing are identified on HRCT in the absence of significant ground glass opacities, the certainty of finding a histologic UIP pattern by SLBx exceeds 96%.10 In fact, when those features are present, the HRCT is considered to display “a radiologic UIP pattern.” Similarly, radiologists often use the term “radiologic NSIP pattern” to describe a HRCT that shows lower zone–predominant ground glass opacities (either alone or in combination with reticular opacities) but no honeycombing. Because of the high degree of accuracy for predicting UIP-pattern histology, when a radiologic UIP pattern is present (in patients with either idiopathic disease or CTD-ILD) on HRCT, SLBx is not performed. However, pattern determination by HRCT is far less accurate for the other, non-UIP, patterns of ILD. In those cases, the decision about whether to biopsy or not, particularly among patients with CTD-ILD (as described above) is less straightforward.
For patients with RA-ILD, a radiologic UIP pattern is a poor prognostic marker. Kim and colleagues studied 82 RA-ILD subjects over a median five years of follow-up. They observed that subjects with a radiologic UIP pattern on HRCT had worse survival than those without radiologic UIP patterns (3.2 years vs 6.6 years, p=0.04).11 In light of these data, the authors have proposed that incorporating knowledge of underlying HRCT pattern in RA-ILD should inform the management approach: Those with RA-NSIP should be treated with immunosuppression and those with RA-UIP should be counseled as to their more unfavorable prognosis and considered for lung transplantation. Additional studies are needed to determine with greater confidence the role of HRCT in predicting prognosis in patients with CTD-ILD.
Defining “CTD-ILD”
In the patient with CTD, determining whether ILD is, in fact, CTD-ILD or ILD of some other etiology is a process of elimination. Alternative etiologies including respiratory infection, medication toxicity, environmental exposure, and recurrent aspiration must be excluded prior to diagnosing CTD-ILD. Complicating matters is the fact that ILD may be the presenting manifestation of CTD, with joint, skin, or other organ involvement appearing months or years after ILD. Nearly 15% of individuals presenting with what is initially diagnosed as idiopathic ILD (i.e., IIP) will eventually develop a classifiable CTD; thus, clinicians should be on the lookout for features of CTD in all their patients with “idiopathic” ILD. Confirming that ILD is a manifestation of CTD may impact management and prognosis thus a thorough assessment for underlying CTD is warranted in patients with idiopathic interstitial pneumonia. Yet there is no standardized approach for such assessments. In our opinion, the evaluation should include a thorough history and physical examination, and measuring for specific autoantibodies (see Table 2). The process can be optimized by encouraging a multidisciplinary approach that includes rheumatologic consultation.