The FDA is considering an application for subcutaneous rituximab and has approved an application for etanercept to treat pediatric patients with plaque psoriasis…


The FDA is considering an application for subcutaneous rituximab and has approved an application for etanercept to treat pediatric patients with plaque psoriasis…
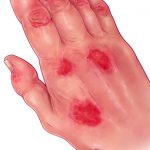
In May, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) published its recommendations for the treatment of psoriatic arthritis (PsA).1 The updated recommendations represent advances in drug development and availability since previous recommendations published in 2009, as well changes in treatment paradigms and the importance of associated aspects of the disease.2…

All athletes—amateur and professional—should understand their risks for developing injury-related arthritis. Rheumatologists and other physicians at the Hospital for Special Surgery in New York take a rapid approach to treating athletes, often considering intense physical therapy, innovative treatments and surgery much sooner than for the average patient—all to keep joints healthy and enable athletes to play for as long as possible…

A study found that ixekizumab decreases disease activity and increases physical function in biologic-naive patients with active psoriatic arthritis…

Adalimumab-atto, a biosimilar to adalimumab (Humira), has been approved by the FDA to treat multiple autoimmune diseases…
Reuters Staff |
(Reuters)—Johnson & Johnson says on Monday that the U.S. Food and Drug Administration approved the company’s psoriasis drug, ustekinumab (Stelara), for use in adults with Crohn’s disease. The drug is approved in the U.S. to treat plaque psoriasis and a type of arthritis associated with psoriasis. Crohn’s is a chronic inflammatory condition in the gastrointestinal…
Reuters Staff |
WASHINGTON (Reuters)—The U.S. Food and Drug Administration on Friday approved a cheaper, biosimilar version of AbbVie’s top-selling arthritis drug, adalimumab (Humira). The drug, adalimumab-atto (Amjevita), is made by biotechnology company Amgen Inc. and was approved to treat rheumatoid arthritis, psoriatic arthritis, Crohn’s disease, psoriasis and other conditions. Amjevita is the fourth biosimilar to be approved…
Reuters Staff |
NEW YORK (Reuters)—Express Scripts Holding, the largest manager of U.S. drug benefits, on Thursday launched a program aimed at tightening spending on drugs for pricey inflammatory conditions such as rheumatoid arthritis. It is the latest Express Scripts effort intended to reduce spending on prescription drugs, such as last week’s announcement of a diabetes program. Early…
Ixekizumab Improves Work Productivity in Patients with Plaque Psoriasis Indirect costs of reduced work productivity can have a significant impact on a patient’s quality of life. A recent article published in JAMA Dermatology analyzed the results of three multicenter, randomized double-blind Phase 3 trials, UNCOVER-1, UNCOVER-2 and UNCOVER-3, which evaluated the effect of ixekizumab on…
A 32-year-old female patient comes in for an initial visit. She is self-referred and complains of pain, numbness and color changes in her fingers when exposed to cold. The patient reports that her right distal index finger, left distal index finger and fourth right finger turn white and blue with pain and numbness when exposed…