On March 22, 2017, Ewa Olech, MD, testified at a hearing before the Nevada State Assembly to voice support for A.B. 245, a bill governing biologic medications and biosimilar substitution in that state. She spoke on behalf of the Rheumatology Association of Nevada (RAN), as its president and founder. The bill establishes guidelines regarding biosimilars and requires…
Search results for: Biologics
The ACR Agenda in D.C.: Where We Stand in Mid-April
Editor’s note: This blog by Dr. Worthing originally appeared on the ACR’s Advocacy Listserv. Here’s a perspective on the current climate in which your government affairs team works. As you read this list of observations, imagine you’re a lawmaker and try to find where the ACR’s agenda fits into the current landscape: Washington is highly…
U.S. FDA Declines to Approve Eli Lilly & Incyte Arthritis Drug
WASHINGTON (Reuters) – The U.S. Food and Drug Administration (FDA) on Friday declined to approve a new drug for rheumatoid arthritis made by Eli Lilly and Co and partner Incyte Corp, the companies said on Friday. The FDA indicated that additional clinical data was needed to determine the most appropriate doses of the drug, baricitinib…

S.C. Rheumatism Society Benefits from ACR Partnership
Editor’s note: This article will be the first in a series of articles highlighting the advocacy work done by state and local societies around the country. The South Carolina Rheumatism Society (SCRS) realizes multiple benefits from its association with the ACR, according to Jeffrey G. Lawson, MD, the society’s treasurer. One example of the support offered:…

Sirukumab Promising for RA
In a clinical trial, RA patients on sirukumab experienced decreased disease activity and improved physical function…
Insurance Subcommittee Responds to Health Plan Complaints
The ACR Insurance Subcommittee (ISC) regularly engages with insurance companies to discuss concerns raised by ACR members and advocate for appropriate coverage and payment policies. The ISC has gotten off to a busy start in 2017, working on a variety of patient access and reimbursement issues. Two recent issues the ISC has taken action on…

Ustekinumab Has Longer Efficacy Duration than TNFIs for Plaque Psoriasis
When compared with TNFIs, ustekinumab demonstrated a longer drug survival rate in patients with severe plaque psoriasis…

Immune System Targeted for Research into New Rheumatoid Arthritis Treatments
WASHINGTON, D.C.—Researchers at the 2016 ACR/ARHP Annual Meeting discussed how they are exploring the immune system in search of groundwork for new rheumatoid arthritis (RA) treatments. The new avenues, supported by the Rheumatology Research Foundation, involve T cell adhesion, new understanding of the role of macrophages and insights into the way IgG glycans function. T…

Factors that Influence Biologic Therapy Choices for Patients with Rheumatoid Arthritis
Recent research analyzed factors influencing the selection of the first-line biologic medications and the real-life factors that lead to switching from those medications to other biologics in treating rheumatoid arthritis (RA). The study compared the use of abatacept and tocilizumab with a tumor necrosis factor alpha inhibitor (TNFi).1,2 Participants were enrolled in the Lombardy Rheumatology…
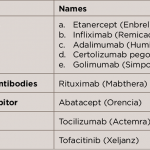
High Cost of Rheumatoid Arthritis Medications Burdens Patients in Saudi Arabia
In the past 15 years, rheumatoid arthritis (RA) has posed an economic burden on patients in Saudi Arabia due to the high cost of the medications used to treat the condition. As a rheumatology consultant, I’ve observed the economic impact on patients in one clinic in a private hospital in Riyadh. RA is a chronic,…
- « Previous Page
- 1
- …
- 44
- 45
- 46
- 47
- 48
- …
- 77
- Next Page »