NEW YORK (Reuters Health)—The oral Janus kinase (JAK) inhibitor tofacitinib appears to improve moderate to severe nail psoriasis, according to a new study. “Nails are hard to treat in psoriasis and we need better treatments,” says Dr. Luis Garza, a dermatologist at Johns Hopkins School of Medicine in Baltimore, who was not involved in the research….
Search results for: Janus kinase inhibitors
U.S. FDA Declines to Approve Eli Lilly & Incyte Arthritis Drug
WASHINGTON (Reuters) – The U.S. Food and Drug Administration (FDA) on Friday declined to approve a new drug for rheumatoid arthritis made by Eli Lilly and Co and partner Incyte Corp, the companies said on Friday. The FDA indicated that additional clinical data was needed to determine the most appropriate doses of the drug, baricitinib…
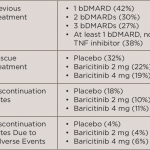
Baricitinib Effective for Treating Refractory Rheumatoid Arthritis
Soon, rheumatologists may have another drug to offer their patients with refractory rheumatoid arthritis (RRA) for whom effective and safe treatment remains challenging. A study published in the New England Journal of Medicine shows that patients with RRA treated with once-daily baricitinib in a 4 mg dose had a significant clinical improvement in symptoms of…

FDA Update: FDA Delays Baricitinib Review & Removes Bupropion & Varenicline Warnings
FDA Review of Baricitinib Delayed The U.S. Food and Drug Administration (FDA) has extended the review period for baricitinib, an investigational medication for treating moderate to severe rheumatoid arthritis (RA).1 Baricitinib is a once-daily oral Janus kinase (JAK) inhibitor currently in clinical studies for inflammatory and autoimmune diseases. The New Drug Application (NDA) for baricitinib…

The OPAL Beyond Study: Tofacitinib Phase 3 Results Positive for Treating PsA
In a recent study, patients with psoriatic arthritis taking tofacitinib had a decrease in disease activity compared with placebo…
Do RA Patients in Clinical Trials for Biologics Represent the Average?
It’s estimated that a majority of patients with rheumatoid arthritis (RA) have been exposed to biologic treatments. However, the randomized controlled trials demonstrating the safety and efficacy of these biologic agents have strict participant eligibility requirements. New research has examined the requirements of 30 trials for biologics and applied those standards to two large clinical cohorts. The result: A majority of these RA patients did not satisfy the criteria…

New Developments in Rheumatoid Arthritis Treatment; Personalized Therapy for Patients Ultimate Goal
SAN FRANCISCO—Considerable progress has been made in the treatment and management of rheumatoid arthritis (RA) in the past two decades, with rheumatologists now able to manage the effects of this chronic, debilitating condition for most of their patients, according to Ronald van Vollenhoven, MD, director of the Amsterdam Rheumatology and Immunology Center (ARC) in the…

Dr. Michael Weinblatt Discusses Current & Future RA Therapies
According to former ACR President Michael Weinblatt, MD, the future of drug therapies for patients with RA rests in the careful and intelligent prescription of current medications and treatment combinations, as well as better patient access through lower costs…
Tofacitinib Not Tied to More Malignancies in RA Patients
NEW YORK (Reuters Health)—The oral Janus kinase inhibitor tofacitinib (Xeljanz, Pfizer) does not increase the risk of malignancies, according to pooled data from more than 5000 rheumatoid arthritis (RA) patients. In an April 22 online paper in Annals of the Rheumatic Diseases, Dr. Lisy Wang of Pfizer, Groton, Connecticut, and colleagues noted that in RA…

The ACR’s State-of-the-Art Clinical Symposium: Experts Discuss Jakinibs, Osteoarthritis, Membranous Lupus Nephritis
CHICAGO—With the approval of the Jak inhibitors (i.e., jakinibs) tofacitinib and ruxolitinib—and others being investigated—rheumatologists need to arm themselves with an understanding of these drugs so they can think critically when evaluating them and deciding how to use them, said John O’Shea, MD, chief of the Molecular Immunology and Inflammation Branch of and scientific director…