PHILADELPHIA—Officials from the U.S. Food & Drug Administration discussed recent drug approvals and drug safety issues at ACR Convergence in November in a session that captured the flurry of activity in the rheumatology sphere at the agency over the past year. Sabiha Khan, MD, clinical reviewer in the Division of Rheumatology and Transplant Medicine at…
Search results for: biosimilars

Cliff Diving: Evergreening & Other Oddities
The glassblowers were in revolt. The island of Murano, in the 13th century, was a perfect home for the glassblowing industry. Connected to Venice through a system of bridges, Murano was surrounded by waters that protected the city from the furnaces that fueled the glassblowers’ craft. The Republic of Venice dominated trade throughout the Mediterranean,…

FDA Approves Riabni, a Rituxumab Biosimilar, to Treat Patients with RA
Based on findings from a double-blind, placebo-controlled study evaluating its efficacy and safety, rituximab-arrx has received FDA approval for the treatment of patients with rheumatoid arthritis.

Rheum After 5: Dr. Jonathan Kay, an Artist at Work
When Jonathan Kay, MD, attends a medical lecture, he does more than just listen to the speakers or watch their presentations. He typically whips out his pen and draws a caricature of someone in the room. Dr. Kay is a professor of medicine and holds the Timothy S. and Elaine L. Peterson Chair in Rheumatology…

Prior Authorization Is Under Review
I just couldn’t believe it. Like all of you, I receive many requests to see patients urgently. And like all of you, I can’t possibly accommodate all of those requests. So I triage: I look through the referrals and try to differentiate patients who want to be seen from those who need to be seen….

Clinical Insights into Axial Spondyloarthritis: Rheumatology Drugs at a Glance, Part 5
Over the past few years, biosimilars and other new drugs have been introduced to treat rheumatic illnesses. Some of the conditions we treat have numerous drug options; others have few or only off-label options. This series, Rheumatology Drugs at a Glance, provides streamlined information on the administration of biologic, biosimilar and other medications used to…

The 2021 ACR Awards of Distinction & Distinguished Fellows
During ACR Convergence 2021 in early November, the ACR honored a group of individuals who have made significant contributions to rheumatology research, education and patient care, announcing the recipients of the ACR’s 2021 Awards of Distinction, as well as the group of Distinguished Fellows. recognized for their contributions. Three pediatric rheumatologists and one pediatric fellow…

FDA Approves First Interchangeable Biosimilar to Adalimumab, Plus a Combination Drug Approved
Cyltezo (adalimumab-adbm) may be interchanged for Humira (adalimumab) for all indications, according to an October decison by the FDA. The FDA has also approved a new combination of celecoxib and tramadol for pain management.
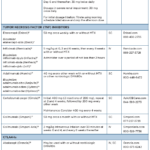
Psoriatic Arthritis Treatment Update
About 30% of patients with psoriasis have psoriatic arthritis (PsA), a complex, multi-faceted, chronic, inflammatory musculoskeletal and skin disease for which the treatment has changed considerably over the past few years.1 Biosimilars and other new drugs have become a therapeutic turning point for many patients suffering from rheumatic illnesses, including PsA. The treatment of PsA…

RheumPAC Advocacy Fund: Effecting Change
Professional practices, state societies and other organizations can support RheumPAC’s work with corporate funds through the RheumPAC Advocacy Fund.
- « Previous Page
- 1
- …
- 9
- 10
- 11
- 12
- 13
- …
- 32
- Next Page »