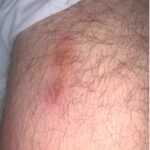
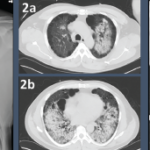
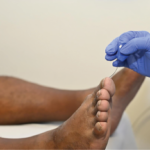
Anti-neutrophil cytoplasmic antibody-associated vasculitis (AAV) is a group of conditions characterized by the development of autoantibodies to the neutrophil proteins proteinase 3 (PR3-ANCA) or myeloperoxidase (MPO-ANCA), leading to systemic inflammation and damage to small blood vessels. AAV includes granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA) and eosinophilic granulomatosis with polyangiitis (EGPA). Review our collection of…