NEW YORK (Reuters Health)—Early oral bisphosphonate use is associated with a lower risk of fractures among oral-glucocorticoid users, researchers from Canada report. Bisphosphonates are commonly used for glucocorticoid-induced osteoporosis, but their efficacy has been established only in primary osteoporosis, where the mechanism of action of bone loss differs from that seen with glucocorticoid use. Dr….
Search results for: osteoporosis

Supplemental Application for Denosumab Goes to FDA
The FDA accepted for review a supplemental biologics license application for denosumab to treat patients with glucocorticoid-induced osteoporosis…

Rheumatologist’s Passion for Gardening Keeps Plants, Patients Healthy
Deborah Dyett Desir, MD, vividly remembers her first day as an undergraduate student at Harvard University, Boston. When her parents helped her move into the dorm, her mother, Betty, handed her a beautiful begonia. “My response was, ‘What on earth am I going to do with this plant?’” she says, recalling how she examined the…

Ethics Forum: A Physician’s Medical Error & the Patient’s Right to Know
Case Ms. A is an 82-year-old woman who presented to the rheumatology office for evaluation of osteoporosis. She had been diagnosed with postmenopausal osteoporosis at age 62 after sustaining a right wrist fracture. She was started on alendronate 70 mg weekly and reported medication compliance. At age 79, she sustained an atraumatic right femur fracture….

New Data May Explain the Role of Sclerostin in Bone Formation
New research in mice shows that sclerostin deficiency may play a significant role in bone formation, possibly despite skeletal age. In the study, sclerostin-deficient mice more readily formed cortical bone and had increases in periosteal bone formation rates, as well as increased expression of the Wnt inhibitor Dkk1, than controls…
What Price Glory (or, at Least, Getting Your Foot in the Door)?
In many respects, this is the beginning of the golden age of rheumatology. Diagnostic and therapeutic approaches are now available that have radically altered for the better the lives of people with diseases that were considered virtually untreatable just a few years ago. The rheumatologist’s approach to patients with rheumatoid arthritis, the spondyloarthropathies, osteoporosis and…
Undetected Fractures Linked to Back Pain in Older Men
(Reuters Health)—About three in five older men with tiny spinal fractures related to osteoporosis reported new or worsening back pain in a new study. Only about one-quarter of new vertebral fractures are diagnosed by a doctor, the study team writes in their September 7 online report in Journal of Bone and Mineral Research, though the…
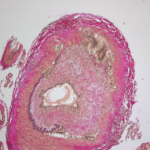
Abatacept Plus Prednisone Therapy Studied for Treating Giant Cell Arteritis
A recent study, conducted by the Vasculitis Clinical Research Consortium and funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), examined whether the addition of abatacept, a drug that affects T cell activation, to standard prednisone treatment could reduce the risk of relapse in patients with giant cell arteritis (GCA).1 Although…

Alendronate Decreases Hip Fracture Risk in Older Patients Using Oral Prednisolone
New research found that older patients on prednisolone who are also treated with alendronate experience a significantly lower risk of hip fracture. Alendronate use was also associated with a lower risk of death…
Switching from Bisphosphonates to Teriparatide May Improve BMD in Women with RA
NEW YORK (Reuters Health)—Switching women with rheumatoid arthritis (RA) from oral bisphosphonates to teriparatide increases bone mineral density (BMD) and trabecular bone score, according to a new report. Researchers in Japan conducted an 18-month observational study of more than 175 women with RA (mean age: 66) who remained on oral bisphosphonates, switched to denosumab (DMAb),…
- « Previous Page
- 1
- …
- 28
- 29
- 30
- 31
- 32
- …
- 60
- Next Page »