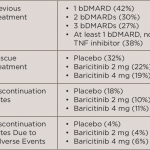
WASHINGTON, D.C.—Rheumatology researchers look for next-generation treatments, healthy interventions, and genetic and microbial clues to disease pathogenesis and therapy response, according to new studies presented at a Nov. 15, 2016, press conference at the 2016 ACR/ARHP Annual Meeting. OA & Physical Function How do you know when a patient with knee osteoarthritis (OA) has the…