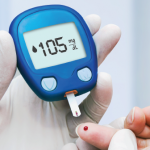
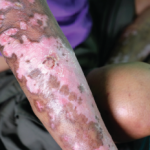
“Wait. I can explain.” One could imagine this phrase coming up under many conditions in daily life. When I first became a physician, however, I would never have expected to use this phrase in my clinic. In medical school, I was taught the importance of dialogue in establishing a relationship with a patient. Statistics indicate…