Laura Hummers, MD, MSc, provided an update on the extra-pulmonary manifestations of scleroderma & the best ways to approach management of these issues.


Laura Hummers, MD, MSc, provided an update on the extra-pulmonary manifestations of scleroderma & the best ways to approach management of these issues.

An update on the state of RA management was presented by Dr. Bryant England, a co-author of the 2021 ACR Guideline on the Management of Rheumatoid Arthrits, during the Review Course held during ACR Convergence 2021.

The Basic and Clinical Research Conference session on Rheumatology Complications of Emerging Viral Infections/SARS-CoV-2 presented findings from numerous studies that help explain some of the idiosyncrasies of COVID-19.

Patrice Fusillo |
During ACR Convergence 2021 in early November, the ACR and the ARP honored a group of distinguished individuals who have made significant contributions to rheumatology research, education and patient care. This month, The Rheumatologist profiles the winners of the ARP President’s and Merit Awards. ARP PRESIDENT’S AWARDS The ARP president can choose to honor ACR/ARP…
Nam D. Nguyen, DO (Maj., USAF, MC), Erica Hill, DO (Lt. Col., USAF, MC), & Jay Higgs, MD (Col. [ret], USAF, MC) |
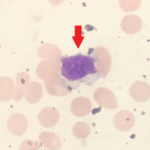
Large granular lymphocytic (LGL) leukemia is a rare, chronic, lymphoproliferative disorder of cytotoxic T cell or natural killer cell lineage with an annual incidence of 0.72 cases per 1 million people in the U.S.1 The most common subtype of LGL leukemia, T-LGL leukemia, follows an indolent disease course and accounts for approximately 85% of cases….
Ashraf Raslan, MD, Dorian Infantino, MD, Roman Zuckerman, DO, & Daniel Berlin, MD |
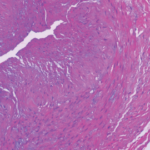
Giant cell arteritis (GCA) is a granulomatous vasculitis of large- and medium-sized arteries, usually affecting the cranial branches of the aortic arch. It is the most common vasculitis, with the highest risk factor being age. Accurate diagnosis and prompt initiation of therapy are of great importance to prevent serious complications, with the most feared being…

Mithu Maheswaranathan, MD |
ACR CONVERGENCE 2021—Rheumatologists are often left in a challenging space when managing medications for patients with rheumatic diseases in relation to contraception, pregnancy and breastfeeding, especially with many novel immunosuppressants and often a dearth of pregnancy safety data. On Nov. 6 during ACR Convergence 2021, leading reproductive health experts came together to speak on this…
Michael Putman, MD |
Andy Abril, MD, a lead author of the ACR/VF guideline for Takayasu arteritis (TAK), discusses the recommendations for TAK.

David R. Karp, MD, PhD |
We are now a year-and-a-half into the COVID‑19 pandemic, and rheumatologists and rheumatology professionals are still facing some of the same challenges that began in spring 2020, as well as new ones. Most recently, we learned that COVID‑19 vaccine efficacy is reduced in some patients on immunosuppressive therapies and the need for additional immunization is…

Data from a single-center registry shines light on 20 years of trends in first-line, biologic disease-modifying anti-rheumatic drug prescriptions for patients with rheumatoid arthritis.