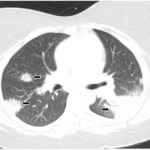
Sarcoidosis is a multisystem disease characterized by noncaseating granulomas in affected tissues, mostly involving the lungs and lymph nodes.1,2 The etiology of sarcoidosis remains unknown but is thought to be due to an inflammatory response to an antigen exposure in genetically predisposed individuals.1 Tumor necrosis factor-α (TNF‑α), a pro-inflammatory cytokine, plays an essential role in…