Dual-energy computed tomography (DECT) is a recently developed advanced imaging technology with a number of clinical applications. For the rheumatologist, the most exciting application is the ability of DECT to specifically detect deposition of monosodium urate (MSU) crystals.
How It Works
Dual-source DECT differs from single-source, single-detector conventional computed tomography (CT) due to simultaneous scanning using two energy sources of different X-ray spectra (80 kV and 140 kV) and two detectors. Evaluation of the different attenuations of the scanned object allows analysis of the chemical composition of materials, which can be color-coded for visualization and measured using automated volume measurement software.
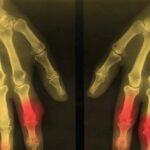
The ability of DECT to detect urate was initially described in the analysis of kidney stones. This imaging method allows noninvasive analysis of the composition of kidney stones, including uric acid stones, with high accuracy. The first case report of DECT for detection of MSU crystal deposits in gout was described in 2007, with a detailed case series of 20 patients with tophaceous gout reported in 2009.1,2 These first reports highlighted the ability of DECT to produce color displays for MSU crystal deposits and to detect deposits that were not apparent on physical examination (see Figure 1).
Practical Use
In clinical practice, a promising role for this technology is in the diagnosis of gout. Gout is a chronic disease of MSU crystal deposition, and microscopic identification of crystals remains the gold standard for diagnosis. However, at times, microscopic diagnosis may not be possible, particularly when synovial fluid cannot be obtained from joints that are difficult to access or due to contra-indications to joint aspiration. Although some clinical presentations of gout are classical (e.g., podagra or subcutaneous tophi), in the absence of microscopic confirmation, diagnosis can be difficult (particularly in the first presentation of an acute flare, or if presenting with chronic gouty arthritis). In this context, noninvasive imaging methods may be of particular benefit for confirming the diagnosis.
A number of studies have examined the diagnostic properties of DECT for gout; these have demonstrated high inter-reader agreement for the presence of MSU crystal deposition using DECT (inter-reader kappa 0.87), with sensitivity of 78–100% and specificity of 97–100%.3-5 The range of sensitivity and specificity in different studies may relate to a number of factors, including what joints were scanned, the number of joints scanned, the analysis protocols used and, most importantly, the stage of disease.
Gout is a chronic disease of MSU crystal deposition, & microscopic identification of crystals remains the gold standard for diagnosis.
Although excellent diagnostic accuracy has been described in people with tophaceous gout, the clinical relevance in this context is limited because this advanced disease stage would not typically require imaging methods for diagnosis. Most studies have not explored the diagnostic accuracy of DECT in people with their first presentation of acute gouty arthritis, or in the context of primary care, where the majority of gout is managed. However, from the limited available data, it appears that in patients presenting with their first episode of acute gouty arthritis, the sensitivity is lower, in the order of 50%.6 The limits of resolution with DECT are 2–3 mm, and DECT cannot detect MSU crystals in fluid <15–20 vol%; therefore, low concentrations or very small aggregates of MSU crystals will not be detected using this method.7
DECT vs. Ultrasound
Ultrasonography (US) is another novel noninvasive method for making the diagnosis of gout. A number of nonspecific features of gout can be observed on US, including synovitis, bone erosion and joint effusion. The characteristic features of gout on US include the snowstorm appearance of synovial fluid, tophi and the double contour sign. This latter finding presents a hyperechoic band over the surface of articular cartilage, which is thought to represent MSU crystals coating the cartilage surface. There is high inter-reader agreement for the US features of tophi and the double-contour sign when assessing acquired images (inter-reader kappa 0.91–1.0). Compared with other forms of arthritis, the US double-contour sign has a sensitivity of 21–100% and a specificity of 95–100%, and the US tophus has a sensitivity of 23–100% and a specificity of 91–100%.
To date, there are very few head-to-head studies comparing the two methods. One recent study compared the diagnostic accuracy of US and DECT in 21 people with a clinical suspicion of gout.5 Similar sensitivity was reported for the two methods, although several false-negative results were reported with DECT that were accurately detected by US.
For some patients, DECT offers some advantages over US, particularly the rapid scanning time (typically one to two minutes for both feet and ankles) and the multiple joints that can be quickly scanned. There are also advantages for using US. For example, unlike US, DECT cannot be performed in the office and requires travel to a dedicated imaging facility. The radiation dose with DECT also limits the frequency of testing, whereas musculoskeletal US has no such limitations.
Other Uses
Another potential role for DECT in clinical practice is assessment of disease complications. Like conventional CT, DECT can detect other abnormalities within the joint, including bone erosion, new bone formation, tendon disease and joint space narrowing, in addition to MSU crystal deposition. This technique allows for the detection of complications of gout, including tendon rupture, carpal tunnel involvement and involvement of the cervical or lumbar spine.
DECT may also have a role in monitoring response to treatment, because automated software allows rapid volume measurement of MSU crystal deposition. Due to the cost and radiation exposure associated with this technology, the principal role of DECT in serial monitoring of MSU crystal deposition is likely to be in the context of clinical trials of new therapeutic agents, rather than in routine clinical practice.
Because tophi consist of both MSU crystals and the host tissue response to these crystals, the volume of MSU crystals within the tophus varies widely, even in tophi of similar physical size. DECT allows measurement of the volume of MSU crystals within a region of interest with excellent inter-reader reproducibility (ICC>0.95) and higher reproducibility than simple physical measurement of tophi.8
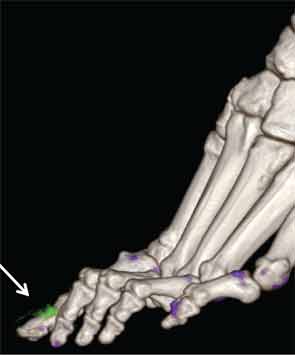
New Insights
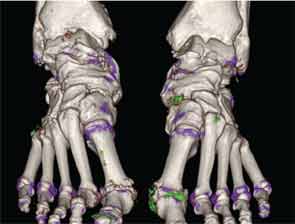
In addition to immediate practical applications within the clinic, DECT has provided insight into patterns and mechanisms of disease in gout by allowing visualization of the pathology of gout in real time. A key early observation: MSU crystal deposition detected by DECT is far greater than that appreciated by physical examination.2 In addition, DECT studies have elucidated the preferential sites of joint involvement in the first MTP joint, midfoot and ankle joints. In the feet, MSU crystal deposition occurs with similar frequency at bone and tendon sites, and certain tendons, such as the Achilles and peroneal, are most often affected (see Figure 2).9 Entheseal involvement, particularly at the Achilles tendon, can also be visualized using DECT.9
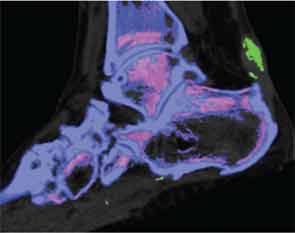
The reasons for such patterns of MSU crystal deposition are currently unknown, and these observations provide a platform for new studies to examine the mechanisms of MSU crystal formation in vivo. DECT has also demonstrated a close relationship between structural joint damage and MSU crystal deposition; implicating interactions between crystals and structures within the joint in the development of bone erosion, new bone formation and cartilage damage in gout (see Figure 3).10
Drawbacks
Despite the utility of DECT, there are some disadvantages and pitfalls that require consideration. First, this technology requires specialized hardware and software, which are costly and not universally available. It requires the use of radiation; the estimated dose is 0.5 mSv per region scanned. Although peripheral regions are usually assessed, frequent repeated measurement is not feasible due to the accumulating risks of medical radiation dosing.
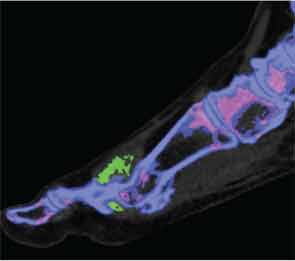
Artifact can distort images at certain sites, such as within thick heel pads and toenails (see Figure 4). Volume assessments should ideally exclude such potential areas of artifact. Technical issues, such as changes in the ratio settings, may substantially alter sensitivity and specificity. The limits of detection of DECT may be lower than ultrasonography, and in particular low concentrations of MSU crystals within joint fluid may not be detected by this imaging method.5,7
Conclusion
Despite these potential limitations, DECT represents an important advance in the clinical management and understanding of gout. Perhaps the most valuable contribution has been the ability to visualize the extent of MSU crystal deposition on DECT scans, even during periods when the patient is not experiencing an acute gout flare. These images emphasize the fundamental concept regarding gout as a chronic disease of MSU crystal deposition. This key concept justifies the central strategy for the effective management of gout, namely the lowering of the serum urate as a way to dissolve crystals. In addition, the pictorial images of the disease provide a clear explanation for prescribing urate-lowering drugs. Thus DECT may serve as a powerful tool for patient understanding and for improving adherence to long-term hypouricemic therapy.
Nicola Dalbeth, MBChB, MD, FRACP, is a rheumatologist and associate professor in the Bone and Joint Research Group, Department of Medicine, University of Auckland, Auckland, New Zealand.
References
- Johnson TR, Weckbach S, Kellner H, et al. Clinical image: Dual-energy computed tomographic molecular imaging of gout. Arthritis Rheum. 2007;56(8):2809.
- Choi HK, Al-Arfaj AM, Eftekhari A, et al. Dual energy computed tomography in tophaceous gout. Ann Rheum Dis. 2009;68(10):1609–1612.
- Glazebrook KN, Guimaraes LS, Murthy NS, et al. Identification of intraarticular and periarticular uric acid crystals with dual-energy CT: Initial evaluation. Radiology. 2011;261(2):516–524.
- Choi HK, Burns LC, Shojania K, et al. Dual energy CT in gout: A prospective validation study. Ann Rheum Dis. 2012;71(9):1466–1471.
- Gruber M, Bodner G, Rath E, et al. Dual-energy computed tomography compared with ultrasound in the diagnosis of gout. Rheumatology (Oxford). 2014;53(1):173–179.
- Manger B, Lell M, Wacker J, et al Detection of periarticular urate deposits with dual energy CT in patients with acute gouty arthritis. Ann Rheum Dis. 2012;71(3):470–472.
- Melzer R, Pauli C, Treumann T, et al. Gout tophus detection—A comparison of dual-energy CT (DECT) and histology. Semin Arthritis Rheum. 2013;Epub ahead of print.
- Dalbeth N, Aati O, Gao A, et al. Assessment of tophus size: A comparison between physical measurement methods and dual-energy computed tomography scanning. J Clin Rheumatol. 2012;18(1):23–27.
- Dalbeth N, Kalluru R, Aati O, et al. Tendon involvement in the feet of patients with gout: A dual-energy CT study. Ann Rheum Dis. 2013;72(9):1545–1548.
- Dalbeth N, Aati O, Kalluru R, et al. Relationship between structural joint damage and urate deposition in gout: A plain radiography and dual-energy CT study. Ann Rheum Dis. 2014;Epub ahead of print.