This case also poses a difficult question for patients and oncologists regarding how they will manage ICI use once an irAE occurs. When an adverse event occurs during ICI therapy, it is important to ascertain if it is from disease progression, an unrelated event or treatment-related irAE. Characterizing these toxicities, even if uncommon, is important.
Management of irAEs is under intense investigation at this point, but general guidelines are being developed. No clear data exist on when to stop ICI therapy or when to reintroduce an ICI once the autoimmune process is managed with steroids for control of responsive cancers.
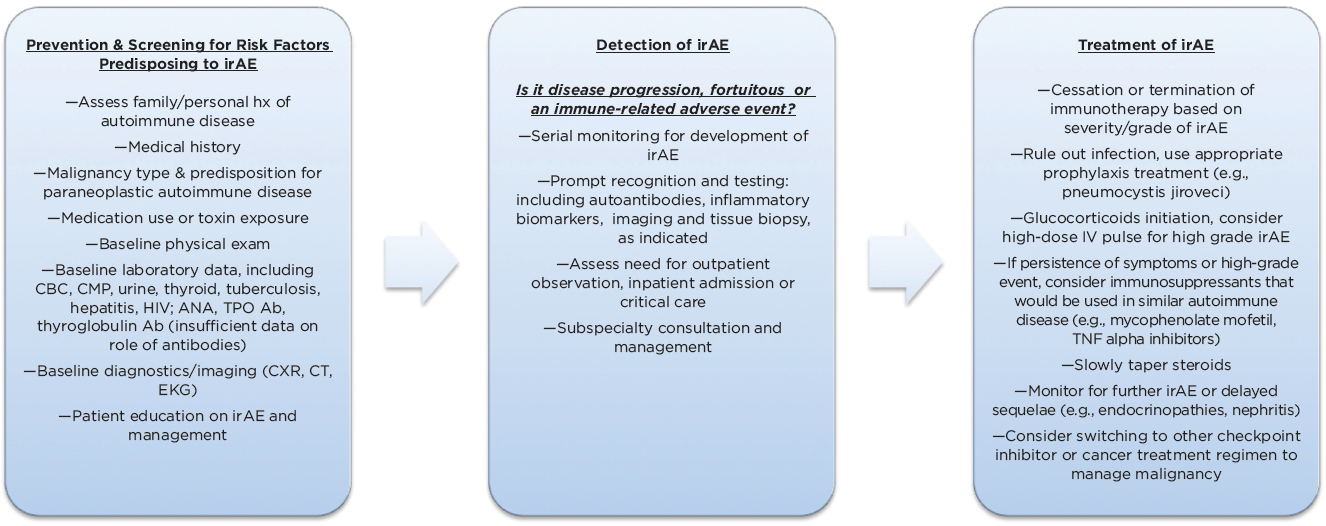
(click for larger image) Figure 3: Management Algorithm for Immune-Related Adverse Events (irAEs) Associated with Checkpoint Inhibitor Use (Adapted)
In the long term, it is unclear how the patient’s underlying immunologic makeup is affected after the use of ICIs or after the development of an autoimmune condition. Further, predictive biomarkers or antibodies that would help identify which patients and organ systems are at risk for immune-mediated toxicity would help target cancer therapy (see Figure 3).2,3,5,25
Immune-related adverse events can involve any organ system, but myalgias, arthralgias, gastrointestinal, dermatologic, hepatic & endocrine autoimmune effects are those most commonly reported with nivolumab use.
Conclusion
This patient had a cardiac history, was on statin therapy and had ovarian cancer treated with the PD-1 inhibitor nivolumab. She developed life-threatening immune-mediated adverse events, including cardiac toxicity, myositis, myasthenia-like syndrome, pulmonary compromise, rash and gastrointestinal toxicity mimicking auto-inflammatory rheumatologic conditions. Immune checkpoint inhibitors have high response rates and overall better tolerance compared with chemotherapy. However, with their increased and broadening uses, it is becoming evident they have a unique inflammatory toxicity innate to the way they affect the immune system with variable presentations. It will be especially important to monitor malignancies often associated with paraneoplastic autoimmune responses themselves in trying to differentiate toxicity of the treatment from manifestation of the cancer.
The questions raised and the management of affected patients requires an interdisciplinary approach. Rheumatologists, oncologists and all subspecialties will need to become familiar and adept at rapidly recognizing and managing irAEs because checkpoint inhibitor use is becoming increasingly prevalent and will likely become the future of cancer therapy.
Priya Chokshi, MD, is a second-year rheumatology fellow at NYU Winthrop Hospital in New York and will be pursuing a career in academic rheumatology.
Roberta Seidman, MD, is an associate professor of pathology at Stony Brook Medical Center in New York. She is board certified in both neuropathology and neurology and has more than 30 years’ experience in evaluation of nerve and muscle biopsies and brain autopsy specimens.