Image Credit: ajt/shutterstock.com
Brentuximab Vedotin Enters Phase 2 Trials
Brentuximab vedotin (Adcetris), an antibody-drug conjugate (ADC) directed at CD30, is currently entering Phase 2 clinical trials for treating systemic lupus erythematosus (SLE).1
The ADC encompasses an anti-CD30 monoclonal antibody, which is attached by a protease-cleavable linker to a microtubule-disrupting agent, known as monomethyl auristatin E (MMAE). The ADC was developed to be stable in the bloodstream, but will liberate MMAE when it is internalized into CD30-expressing cells. This Phase 2 trial will help assess the safety and efficacy of brentuximab vedotin in managing adults with SLE. The drug is currently being investigated in at least 30 clinical trials, including four Phase 3 trials for different oncology uses, such as CD30-positive lymphomas.
FDA Reviews Xeljanz Application
The U.S. Food and Drug Administration (FDA) has accepted for review a new drug application for tofacitinib citrate (Xeljanz) 11 mg once-daily modified release tablets to treat moderate to severe rheumatoid arthritis in patients who have had an inadequate response or demonstrated intolerance to methotrexate.2 Preliminary equivalency data have shown comparability between tofacitinib 11 mg once daily and tofacitinib 5 mg twice daily, the current FDA-approved dose. The FDA is also reviewing tofacitinib 10 mg and 5 mg twice-daily tablets for treating adults with moderate to severe chronic plaque psoriasis who are candidates for systemic therapy or phototherapy. Tofacitinib is an oral Janus kinase inhibitor.
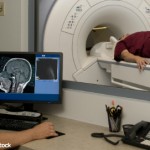
FDA Investigates MRI Safety after Brain Deposits of GBCA Found
The FDA is investigating the risk of brain deposits developing after repeated use of gadolinium-based contrast agents (GBCAs) for magnetic resonance imaging (MRI).3 Recent medical publications have reported that GBCA deposits have remained in patient brains long after the last administration of these agents, particularly in patients who have undergone at least four MRI scans. It is not known whether effects from the gadolinium deposits are harmful or if they can lead to adverse health effects.
The FDA is continuing to study any potential safety risks. No current changes to the product labeling for GBCAs are being recommended at this time.
To prevent the potential accumulation of gadolinium, the FDA is requesting that healthcare professionals limit GBCA use to clinical circumstances in which the additional information provided by the contrast is necessary. The FDA is also requesting that healthcare professionals reassess the necessity of repetitive GBCA MRIs in established treatment protocols.
In published studies, investigators reviewed noncontrast MRIs of patients who had received several GBCA MRIs as part of cancer management, multiple sclerosis management or for other illnesses. The noncontrast MRIs demonstrated findings that suggested gadolinium contrast was being retained in various brain structures. So far there have been no signs or symptoms of adverse health effects and no pathological changes associated with these gadolinium brain deposits. In some of these studies, brain examination at autopsy confirmed gadolinium deposits.