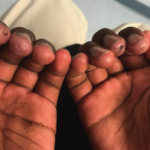
Ho et al. found that upadacitinib may impede the progression of bone erosion in patients with RA. Additionally, bone scans of patients with limited exposure to conventional synthetic disease-modifying antirheumatic drugs showed bone erosion regression, which may result from upadacitinib’s inhibition of Janus kinase 1.