Two JAK inhibitors, one recently approved by the FDA, have shown improvements in patients with active RA for whom other therapies have proved ineffective…


Two JAK inhibitors, one recently approved by the FDA, have shown improvements in patients with active RA for whom other therapies have proved ineffective…

Mary Choy, PharmD, BCGP, FASHP |
Over the past few years, biosimilars and other new drugs have been introduced to treat rheumatic illnesses. Some of the conditions we treat have numerous drug options, others have few or only off-label options. This series, “Rheumatology Drugs at a Glance,” provides streamlined information on the administration of biologic, biosimilar and small molecule inhibitor drugs…
If approved by the U.S. Food and Drug Administration (FDA), difficult-to-treat patients with axial spondyloarthritis who fail or are intolerant to standard treatment with tumor necrosis factor inhibitors (TNFi) may have a new treatment option. That new option is a high-affinity monoclonal antibody, called ixekizumab, which selectively targets an area linked to the immunopathology of…

Paul H. Caldron, DO, PhD, MBA, & John R.P. Tesser, MD |
As we turn the corner on the second decade of biologic use for rheumatic disorders, a reappraisal of approach in our communication with patients is due. In practice, the impact these agents have on patients’ lives justifies the friction rheumatologists face when connecting patients to them. You can understand why older rheumatologists who apprenticed on…
Gbemisola Olayemi, MD, Evangeline Scopelitis, MD, & Jerald M. Zakem, MD |
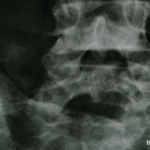
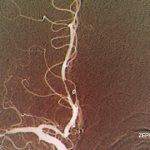
Vasculitis is a group of chronic inflammatory diseases in which the blood vessel is the target of an immune reaction. They can be secondary to connective tissue disease, idiopathic or due to infection, neoplasm or drugs.1 Primary angiitis of the central nervous system (PACNS) is a rare syndrome characterized by inflammatory cell infiltration and necrosis…

The FDA has approved Truxima (rituximab-abbs), which is biosimilar to Rituxan (rituximab), for treating adults with CD20-positive, B-cell non-Hodgkin’s lymphoma…

New research underscores the possibility that interleukin (IL) 12, IL-23 or both play roles in the immunopathology of SLE. In the study, when added to standard-of-care treatment for active SLE, ustekinumab demonstrated better efficacy than placebo and had a safety profile consistent with that of ustekinumab therapy in other diseases…

AMSTERDAM—With new therapies coming into the marketplace, researchers are working to tease out the risk of infection for rheumatoid arthritis (RA) patients. Existing data suggest the risk of infections—even fatal ones—is real. But over time, improvements have taken hold, particularly for tuberculosis, according to an infectious disease expert at EULAR: the Annual European Congress of…

Due to a range of factors, determining the precise infection risk posed by new biologic therapies to RA patients is difficult. But progress has been made and health registries may be helpful, said Olivier Lortholary, MD, PhD, during the 2018 EULAR: Annual European Congress of Rheumatology…

New research shows tanezumab may be safe and effective for patients with osteoarthritis pain…