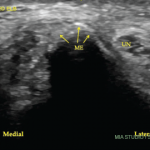
A 27-year-old, left-handed man was referred to our ultrasound clinic for left elbow pain. History The patient had been a pitcher on a Minor League Baseball team. Two years before, he developed sudden, severe medial elbow pain while pitching in a game. The pain was associated with some tingling down the left medial forearm. The…