The FDA has designated upadacitinib a breakthrough therapy to treat adults with moderate to severe atopic dermatitis…


The FDA has designated upadacitinib a breakthrough therapy to treat adults with moderate to severe atopic dermatitis…

SAN DIEGO—In November, the Rheumatology Research Foundation honored 155 award and scholarship recipients at the Awards Celebration, an annual luncheon, held in conjunction with the ACR/ARHP Annual Meeting. The event celebrates the accomplishments of rheumatology professionals who have received funding from the Foundation. In congratulating the award recipients, executive director Mary Wheatley, CAE, IOM, emphasized…

Mysterious Ways The juxtaposition of the old and the new was readily evident that busy Wednesday morning. My first patient, a 94-year-old gentleman, Hal, arrived with a precise request. His rheumatologist for the past 40 years had just retired, and he was searching for a doctor with expertise in the use of gold sodium aurothioglucose,…
Will Boggs MD |
NEW YORK (Reuters Health)—Most recent clinical practice guidelines (CPGs) from the American College of Rheumatology (ACR) are based on expert opinion and lack A-level evidence in support of their recommendations, researchers report. “I’d like to highlight not just for providers but also for patients and policymakers that, even though we in the United States are…

In a Year in Review session at the 2017 ACR/ARHP Annual Meeting, Daniel Solomon, MD, MPH, highlighted the latest and most intriguing aspects of clinical research on rheumatic diseases from 2017. His discussion touched on medical therapy, genetics, the effects of bariatric surgery and diet, cancer risk and more…

FDA Approves Adalimumab-adbm On Aug. 29, the FDA approved Cyltezo (adalimumab-adbm), a biosimilar to Humira (adalimumab).1 Cyltezo was approved as a prefilled syringe to treat multiple chronic inflammatory diseases, including moderate to severe active RA, active psoriatic arthritis and ankylosing spondylitis, and moderate to severe plaque psoriasis. The treatment has also been approved for moderate…

Patients who receive abaloparatide and switch to alendronate have a statistically significant reduction in fracture risk through 3.5 years, according to a new study…
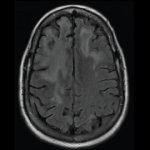
Eugene Jalbert, DO, MBA, Priyanka Murali, DO, Rakhee Shah, DO, Robert DiGiovanni, DO, FACOI, FACR, & Rubaiya Mallay, DO, FACOI, FACR |
Rheumatoid arthritis (RA) is a systemic autoimmune disease associated with erosive destruction of diarthrodial joints. Patients who are seropositive are more prone to developing extra-articular manifestations, such as rheumatoid lung, rheumatoid nodules and others. With the development of disease-modifying anti-rheumatic drugs (DMARDs), the incidence and severity of these extra-articular manifestations has declined. Below, we describe…
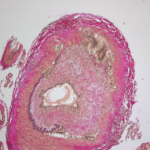
A recent study, conducted by the Vasculitis Clinical Research Consortium and funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), examined whether the addition of abatacept, a drug that affects T cell activation, to standard prednisone treatment could reduce the risk of relapse in patients with giant cell arteritis (GCA).1 Although…

The FDA has approved adalimumab-adbm, a biosimilar, to treat multiple chronic inflammatory diseases…