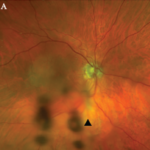
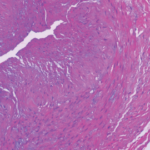
ACR CONVERGENCE 2021—Many of the effects of childhood-onset systemic lupus erythematosus (cSLE) and vasculitis carry into adulthood and present adult rheumatologists with key differences in managing these patients after their transition from a pediatric to an adult provider. “The young adult with childhood-onset lupus is similar in many ways to adults with lupus, but there…