(Reuters Health)—U.S. commercial health plans only covered biosimilar treatments as preferred products in 14% of coverage decisions last year, according to an analysis of publicly available data on coverage decisions.1 Researchers examined records from the Tufts Medical Center Specialty Drug Evidence and Coverage (SPEC) database, which has information on coverage decisions made by 17 of…

Rheumatology Biologic Expertise Valued for COVID-19 Treatment Decisions
At the Loma Linda University Medical Center, Calif., rheumatologists play a key consulting role for COVID-19 patients who may benefit from the use of biologic treatments…

Rheumatoid Arthritis Therapy Update: What’s Changed & What’s the Same
SNOWMASS VILLAGE, COLO.—Current trends in rheumatoid arthritis (RA) therapy are the increased use of newer medication categories, such as Janus kinase (JAK) inhibitors (Jakinibs) and biologics, and the rising costs of treatment. Unchanged is the consistent use of methotrexate as an effective therapy. These topics and more were discussed at the ACR Winter Symposium during…
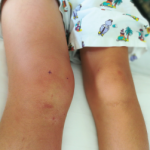
Pediatric Cases Require Special Considerations & Aggressive Treatment Plans
ATLANTA—Managing pediatric patients with rheumatic disease involves special considerations, such as developmental concerns and physiological traits that may affect dosing of medications, according to two experts. During a session at the 2019 ACR/ARP Annual Meeting, Courtney Kremer, ARNP, a pediatric nurse practitioner at the University of Iowa Stead Family Children’s Hospital, Iowa City, and Jessica…
Clinicians Discuss Current & Future Rheumatoid Arthritis Approaches
ATLANTA—When it comes to treating rheumatoid arthritis (RA) patients, most clinicians agree: One size does not fit all. Many treatment options exist, and seldom is there 100% consensus on what the first course of action or general approach should be. In the face of such variability, four clinicians took the stage at the 2019 ACR/ARP…

FDA Update: New Drug Approvals, New & Expanded Indications, & More
ATLANTA—New drug approvals, new and expanded drug indications, and important safety and other updates relevant for rheumatologists were presented by three physicians from the U.S. Food & Drug Administration (FDA) on Nov. 11 at the 2019 ACR/ARP Annual Meeting. New JAK Inhibitor Approved for RA On Aug. 16, 2019, the FDA approved upadacitinib (Rinvoq), an…

Remembering Etanercept & the Advent of the Biologic Era
As a veteran rheumatologist, I remember the clinical trials of etanercept’s (Enbrel’s) efficacy. And when the drug was first approved in 1998, I participated in those clinical trials and realized the effectiveness was astonishing. It was easy to tell which patients were treated with etanercept vs. those who received placebo, even though both groups were…
Biologic Spending & Price Trends
Any given rheumatology patient who needs a biologic disease-modifying anti-rheumatic drug (DMARD) will spend $22,000–44,000 on their medication each year…

Canada & E.U. Approve Upadacitinib for RA
Upadacitinib will soon be available to treat patients with moderate to severe rheumatoid arthritis in Canada and the E.U…

EU Approves Remsima SC, a Biosimilar to Infliximab
Subcutaneous CT-P13 (Remsima SC), biosimilar to infliximab, will soon be available in the E.U. to treat adults with rheumatoid arthritis…
- « Previous Page
- 1
- …
- 11
- 12
- 13
- 14
- 15
- …
- 44
- Next Page »