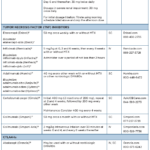
We are fortunate to have clinical practice guidelines for the management of psoriasis and psoriatic arthritis (PsA) from multiple organizations to help navigate today’s rapidly evolving therapeutic landscape. We are further fortunate to have multiple specialists to manage these conditions: rheumatologists and dermatologists. However, multiple guidelines, multiple drugs and multiple specialists can create a paradox…