Rituximab-abbs, which is biosimilar to rituximab, is now available in the U.S. to treat specific cancers…


Rituximab-abbs, which is biosimilar to rituximab, is now available in the U.S. to treat specific cancers…

Intravenous rituximab can now be used to treat pediatric patients with GPA and MPA as young as two years old…

Recent research into rituximab-induced serum sickness (RISS) reinforces the understanding that patients generally recover quickly. However, the study also found patients with autoimmune disease, especially SLE, had a significantly higher risk for developing RISS than patients using rituximab to treat hematological malignancy…
A 66-year-old female patient returns for a second infusion of rituximab for her diagnosis of rheumatoid arthritis in multiple sites. She is rheumatoid factor positive. She says the pain in her knees, elbows and neck has slightly improved. She rates the severity of her pain at a 7 on a 10-point scale, which is an…
Take the challenge. CPT Codes: 96413, 96415 x 3, J9312 x 5, 96375, J2920 Diagnoses: M05.79 Coding Rationale As of Jan. 1, 2019, the Healthcare Common Procedure Coding System (HCPCS) code for rituximab was changed from J9310 rituximab 100 mg, to the new HCPCS code J9312 (injection, rituximab, 100 mg). According to a Verywell Health…

The FDA has approved Truxima (rituximab-abbs), which is biosimilar to Rituxan (rituximab), for treating adults with CD20-positive, B-cell non-Hodgkin’s lymphoma…
Researchers sought to identify predictors of patients with anti-neutrophil cytoplasmic antibody (ANCA) associated vasculitis (AAV) who took rituximab for maintenance had a better sustained remission rate through 60 months than those taking azathioprine, according to the latest results from the maintenance of remission using rituximab in systemic ANCA-associated vasculitis (MAINRITSAN) trial, a prospective, randomized trial…
Rheumatologists prescribe rituximab for induction and maintenance treatment for anti-neutrophil cytoplasm antibody (ANCA) associated vasculitides (AAV). Maintenance treatment typically employs fixed-schedule dosing, but in the recent maintenance of remission using rituximab in systemic ANCA-associated vasculitis II (MAINRITSAN2) trial, researchers from the French Vasculitis Study Group examined whether individually tailored maintenance dosing might work better. “The…

The FDA has approved an update for rituximab’s label, which will include safety and efficacy information for treating ANCA-associated vasculitis…
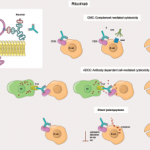
Severe infections occurred in 25% of patients with anti-neutrophil cytoplasm antibody (ANCA) associated vasculitis (AAV) who were treated with rituximab, according to results of an observational study conducted in the United Kingdom (U.K.) and Austria. Trimethoprim-sulfamethoxazole (TMP/SMX) prophylaxis, however, reduced the risk of severe infections, the investigators reported in Annals of the Rheumatic Diseases.1 Andreas…